Collections, Knowledge and Engagement
- GR-3346.37 2021 Speech from the Throne
- The archives’ most recent record, created less than a year ago·
- GR-3518 Records of the Provincial Health Officer *
- This accrual covers the records of Dr. Perry Kendall, who served as the Provincial Health Officer from 1999 to 2018. The records address the health of Indigenous populations, water quality and pandemic influenza preparation among other public health topics.
- GR-3676 and GR-3677 Cabinet committee records and Premiers’ records*
- Large accruals were added to these series this year, documenting cabinet proceedings and priorities. Most records up to 1991 are publicly accessible.
- GR-3890 Drafting legislation case files*
- Comprehensive records covering all aspects of the process for drafting and approving provincial legislation. The records relate primarily to the 1970s and 1980s.
- GR-3948 Notebook related to the Tsilhqot’in War
- This delicate and difficult to decipher notebook relates to the events of the 1864 Tsilhqot’in War. It includes a diary of a group of settlers attempting to apprehend the Indigenous men allegedly involved in the deaths of several settlers.
- GR-3996 Conservation Officer Service major investigation case files*
- The series consists of the major investigation case files of the Conservation Officer Service between 1992 and 2007. Major cases are serious in nature and address complex issues such as trafficking animal parts, big-game poaching, illegal fishing or guiding, or selling animals for human consumption that are procured illegally.
- GR-3999 Environmental Appeal Board records*
- This series includes appeal files submitted to the Environmental Appeal Board and its predecessors: the Pollution Control Board and Pesticide Control Board. The records document the government’s management of the environment.
- GR-4000 Provincial Police circulars and wanted posters*
- This series includes a variety of informational posters for alleged criminals wanted across Canada and the United States. Many of the records are pasted into indexed volumes, serving as simple criminal record “databases.”
- GR-4002 Forest Practices Board meeting files*
- This series includes records of the Forest Practices Board which serves as the independent watchdog for sound forest and range practices in the province.
- GR-4005 Conservation Officer Service wildlife attack final reports*
- This series consists of final reports summarizing wildlife attacks on humans created by the Conservation Officer Service between 1991 and 2012. They illustrate the evolution of wildlife attack investigative technique, causes of wildlife attacks, and methods used to dispatch wildlife.
- GR-4008 BC Ambulance Service and air evacuation major accident and investigation files*
- This series consists of BC Ambulance Service (BCAS) and air evacuation major accident and incident investigation files between 1992 and 2011.
- GR-4041 Witness statements from CPR train robbery
- These records purport to be related to a train holdup by the infamous Bill Miner at Ducks. The details match other historical accounts of the incident; however, the year is wrong! These may benefit from some further research.
- GR-4048 Prince Rupert Forest District wild fire mapping records
- These huge volumes document wildfires in the Prince Rupert region from 1921 to 1980
- GR-4051 British Columbia Lottery Corporation’s ombudsperson’s investigations*
- This series consists of the ombudsperson’s investigation into the British Columbia Lottery Corporation’s prize payout procedures between 1999 and 2010. BCLC retailers and BCLC retailer employees appeared to be winning major prizes at a higher rate than other players in the province. The public and the media expressed concern that some of those winning tickets may actually belong to ordinary players.
- GR-4063 Cariboo government office records
- This series contains colonial records from the Cariboo region, including Barkerville. Records cover all aspects of government administration in the area from registering mining claims to court cases to crime statistics.
- GR-4064 Correspondence regarding immigration
- This series includes correspondence of the Provincial Immigration Officer from 1903-1905. Many records relate to the deportation of Japanese people under the racist 1904 Immigration Act.
- 24930E Plan of the No. 2 Extension coal mine
- This plan was made as part of the investigation into the cause of a 1909 explosion in the Ladysmith mine, resulting in the deaths of 32 men.
- GR-0522 Kamloops Government Agent land records
- This is a large series of records (160 boxes) that show a comprehensive history of land use in the Kamloops area over 100 years. This includes the time period the land was managed by the Canadian government as part of the Railway Belt.
- GR-3929 Riverview Hospital Historical Collection*
- This collection includes a wide variety of records related to the operation of Riverview Mental Hospital (previously known as Essondale). The records were collected and maintained by the Riverview Historical Society. With the closure of the hospital in 2012, government records in the society’s holdings were transferred back to the government and have recently been made available in the archives. The collection includes records of all media and provides an overview of the hospital’s history, grounds, staff, and patient care.
- 10am-12pm, Tuesday August 24th – teachers from SD61, 62, 63 or 79 (onsite at the museum)
- 10:30-12pm, Wednesday August 25th – teachers in districts beyond Greater Victoria region (online format)
- aSchool of Biology, Faculty of Biological Sciences, University of Leeds, Leeds, U.K.
bRoyal British Columbia Museum, Victoria, BC, Canada - A partial backing means that the painting is not supported evenly, which often leads to folds, creases and other types of damage if the work is not handled very carefully
- Differential support can also lead to deformations in the paper because some areas of the sheet are constricted from movement (with environmental fluctuations) and some aren’t, leading to a build-up of internal stresses
- The poor-quality cardboard was also very brittle and likely to crack or snap. If a snap in the cardboard were to happen, this would inevitably translate into bends, creases or even breaks in the painting as well.
- Acidic components of the poor quality wood pulp cardboard (as well as acidic components that appear to have been absorbed from another source, visible as brown lines across the cardboard backing) will leach into the painting causing the paper to degrade and become brittle and discoloured. This could eventually affect the paint as well.
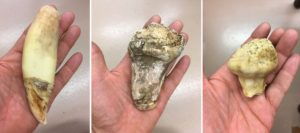
As the museum and archives prepare to move the collections to the new Collections and Research Building in Colwood, BC, more items are arriving in the Conservation labs for treatment to ensure they are stable enough to endure the journey. This past year, one portrait made its way to the paper conservation lab in need of pre-move stabilization. In this case, a large hole and obvious moisture damage were cause for concern.
This portrait of Mr. and Mrs. Lum (BC Archives, J-01350) is one in a collection of four (BC Archives, MS-3411). Mr. Chin Lum Kee, also known as Ah Lum, was born in Guangdong (previously known as Canton), China in 1835. He arrived in the new colony of British Columbia during the Fraser River Gold Rush. While in Sto:lo territory, he met his wife, Squeetlewood, also known as Lucy, who was born in 1854.
The portrait is a solar enlargement, based on a salted paper print (more information on this type of photography below). Black and white crayon have been used to work up the figures’ clothing and watercolour has been used to add soft colouring to the faces and background. In keeping with photographic portraiture of the time, the figures are captured from the chest upward with their bodies fading softly into the bottom of the picture plane. This solar enlargement on paper has been mounted to canvas and stretched over a wooden strainer.
A Brief History of Solar Enlargements
Solar enlargements, also known as crayon portraits, were produced between the 1860s and the early 20th century. During the 19th century, photographic prints were made by placing photo-sensitised paper in direct contact with a photographic negative. Making a photographic print larger than the original negative began to be possible in the late 1850s with the invention of “solar cameras”. These enlarged prints required lengthy exposures and were faint and soft-focused. Furthermore, any imperfections in the photographic negative would be amplified in the enlargement.
As a result, these faint photographs were used as a sort of under-drawing upon which charcoal and coloured paints and crayons were used to retouch and enhance the image. It was common for artists to be employed alongside photographers for this purpose.
The most popular process for these enlarged photographic “under-drawings” was the salted paper print, however albumen prints could be used as well. Bromide enlarging paper was introduced in the 1880s which employed a gelatin emulsion layer available in many different surface textures. For more information on historical photographic processes, visit the Image Permanence Institute’s Graphic Atlas.
Condition Issues
The portrait of Mr. and Mrs. Lum came to the conservation lab because it was not in a condition where it could be safely handled. There was a large hole in both the paper photograph and the canvas behind it. The paper had been worn away along the left edge, resulting in a loss of some of the image. This is likely, at least in part, the result of some previous moisture damage. There were also tideline stains across the image from previous moisture damage. The adhesive holding the paper photograph to the canvas was disintegrating (or previously dissolved?) which meant most of the photograph was not actually attached to the stretched canvas support and was therefore more vulnerable to creases, rips and tears. Finally, the whole structure was heavily soiled with dirt, dust and debris.

Figure 3. Bottom edge of [Mr. & Mrs. Lum], before treatment. Photograph lifted to show underside of primary support (paper) and the secondary support (canvas).
Treatment
Treating a composite object is always difficult. Since this portrait was so heavily soiled and already stained by moisture damage, an aqueous wash (water bath) was deemed necessary. The decision was made to disassemble the object, which meant removing the partially attached photograph (or “primary support”) from the stretched canvas and then removing the canvas from the strainer. Each piece was then cleaned separately and reassembled once dry.
Cleaning the Primary Support
To be sure none of the media would be affected by washing, solubility testing of all the different media types as well as the staining was carried out under the microscope. The media was already thought to be quite stable—after all, it had survived moisture damage in the past—and the solubility tests proved this to be the case. The tidelines had mixed results with some appearing quite soluble and others less so. These results were enough to suggest that the primary support would benefit from a wash: the media would not be affected and the stains would likely disappear or at the very least, be reduced. Washing out these stains and other products created as a result of deterioration in the paper would also raise the pH, making the paper more chemically stable.

Figure 4. Recto of [Mr. & Mrs. Lum], before treatment. Paper triangles point to areas where solubility testing was carried out under the microscope.
Then, the solar enlargement was washed in an aqueous bath. There were some small fragments that had come detached from the left side of the photograph. These fragments were also washed but using a different, more delicate method. These fragments were gently placed on wet Tekwipe®, a non-woven fabric made from cellulose and polyester. This fabric has capillaries that draw clean water from one basin through the fabric, wetting out the paper resting on its surface and removing dirt and other products of deterioration in the paper and then move the dirty water along the rest of the fabric to the empty basin on the other side.

Figure 6. Primary support being removed from aqueous bath. Note the colour of the wash water has turned yellow with dirt and the water-soluble products that are formed as paper deteriorates.

Figure 7. Small fragments from the left side of the primary support being washed on Tekwipe® using capillary action.
Once washed, all pieces for the primary support were placed under weight to dry flat. After a few days of drying, the photograph was humidified and a thin piece of Japanese tissue paper was applied to the back. This lining provides a consistent support layer and prevents any further losses or tears around the areas that have already suffered losses.

Figure 8. Recto of solar enlargement after aqueous wash and lining with Japanese tissue. Note that some tidelines remain but they are reduced. The staining across Mrs. Lum’s face has been washed away.
Cleaning the Canvas
The canvas was gently removed from the wooden strainer and vacuumed on very low suction to remove loose clumps of dust and dirt that were deposited on the surface, particularly along the perimeter where the strainer had been.
The canvas was also heavily soiled and need a wash, however there is an inscription in blue ink on the back of the canvas that was discovered to be water-soluble during the solubility testing mentioned previously. To protect that ink from being solubilized during the wash, cyclododecane was applied as a temporary seal. Cyclododecane is a waxy substance that becomes liquid when warmed. It was applied over the ink as a warm liquid and solidified in place upon returning to room temperature. One of the unique properties of this wax is that it sublimes (passes directly from a solid state to a gas state) at room temperature over relatively short periods of time.
With the ink inscription temporarily sealed with cyclododecane, the canvas was washed with the help of textiles conservator Colleen Wilson. After the wash, it was allowed to dry and the cyclododecane was allowed to sublime, leaving the ink behind, unaltered.

Figure 9. Paper conservator, Lauren Buttle, applying cyclododecane to canvas to temporarily seal the water-soluble inscription in preparation for an aqueous wash.

Figure 10. Verso of canvas after being removed from strainer, with cyclododecane applied to inscription, before aqueous wash.

Figure 11. Textiles conservator, Colleen Wilson, blotting canvas with a sponge during first bath with detergents.

Figure 12. Verso of canvas 1.5 weeks after aqueous wash and after cyclododecane has fully disappeared.
Cleaning the Strainer
The wooden strainer was also vacuumed to remove surface dirt and dust. Some residual adhesive left on the outside edges of the strainer were re-moistened and removed with a Teflon spatula.
Reassembly
With all the individual pieces cleaned, the lined photograph was reattached to the canvas and then the canvas was re-stretched and adhered to the outside edges of the strainer. The Japanese tissue lining of the primary support provides support over areas of loss.
Re-Housing the Lum Portraits
While the main reason for diverting the Lum portraits to the conservation lab was to stabilize them for the move, there was also the issue of how to safely transport them. When they arrived in the lab, they were altogether in a paper folder, a housing system that was not offering sufficient protection in the context of a collection move.
Once conserved, this portrait, as well as the other three portraits in the collection, were placed in HTS (handling-travel-storage) frames. These storage structures are based on models developed by the Canadian Conservation Institute and the National Gallery of Canada. These frames will provide protection to each work and can be easily re-used for other similarly-sized works if necessary.
References
Canadian Conservation Institute (2018). “Framing a Painting – CCI Note 10/8”. Government of Canada. Framing a Painting – Canadian Conservation Institute (CCI) Notes 10/8 – Canada.ca
Reilly, James M. (1986). Care and Identification of 19th-Century Photographic Prints. Rochester, NY: Eastman Kodak Co.
Whitman, Katharine (2005). “The Technology of Solar Enlargements”, Topics in Photographic Preservation, Vol. 11, pages 104-110. Washington DC: American Institute for Conservation of Historic & Artistic Works. History (culturalheritage.org)
A few months ago, a box of archival records that had been requested for access was transferred to the Royal BC Museum paper conservation lab. These documents were part of the Archibald Menzies fonds, a small collection of records and belongings related to Archibald Menzies (1754–1842), a Scottish surgeon and naturalist who accompanied Captain George Vancouver aboard the HMS Discovery in 1791–95.
The documents in this box were mostly parchment and had been folded several times in the past, then piled into a box for storage. Upon retrieving the box, the archivist realised that none of these documents were easy to open. The parchment was hard and inflexible, suggesting that any attempt to forcibly flatten out the records might lead to damage. When problems like this arise, these materials are diverted to our conservation team for assessment and remedy.
So, how can we convince parchment to relax, open up and share its hidden secrets? First, we need to understand where it’s coming from.
The Nature of Parchment
Parchment is made from animal skin—usually that of a calf, sheep or goat. The skins are removed from the animal, dehaired and defleshed, and then treated with a lime or other alkali solution. After liming, the skin is dried under tension, and in some cases, surface treatments are carried out to make it more suitable to receive inks and paints. Before the invention of paper, parchment was a common writing support in Europe and parts of the Middle East. It continued to be used as such for several centuries after papermaking became popular and widespread.
Parchment is very sensitive to moisture and heat. This means that when water is present, the parchment will expand and absorb moisture. When conditions become warm and dry, the parchment will shrink and release moisture. Over long stretches of time, these cycles of shrinking and expanding cause the parchment to deteriorate, becoming distorted and hard.
Relax and Unfold
The parchment documents in this collection were tightly folded, and attempts to open them were met with strong resistance. Forcing them open could have potentially led to cracks, breaks and losses in the document; however, leaving them as they were meant that no one could read them.
To open these documents without incurring damage and loss, we needed them to relax. This state of inner peace was achieved with the careful introduction of moisture. Since parchment is so sensitive to moisture, the use of moisture to treat deteriorated parchment might seem counterintuitive; however, in the case of this treatment, moisture is delivered in the form of water vapour (never liquid water), and it was delivered with control.
The tightly folded documents were placed in an environmental chamber where the relative humidity (RH) could be raised using an ultrasonic humidifier. The RH levels were monitored throughout this treatment using a hygrometer placed in the chamber. Slowly, over a period of approximately two hours, the documents were unfolded bit by bit, until they were relaxed enough to fall completely open.
Once the documents had fully revealed themselves, they were placed under very gentle pressure between felt pads to ensure they dried flat.
A New Home
Now that these documents are open for viewing, they will be stored flat. They have been transferred to a flat storage box with custom padded trays for two of the documents, with seals and cords attached, ready for the next researcher.
Every year the Government Records team at the BC Archives processes over 2,000 boxes of records created by the provincial government. Most of these records can now be accessed by anyone, even you! Just remember that all government records are covered by privacy legislation, such as the Freedom of Information and Protection of Privacy Act. This means some records have access restrictions on sensitive or personal information. Here is an overview of just a few of the record series from the last year or so that are now available in our database.
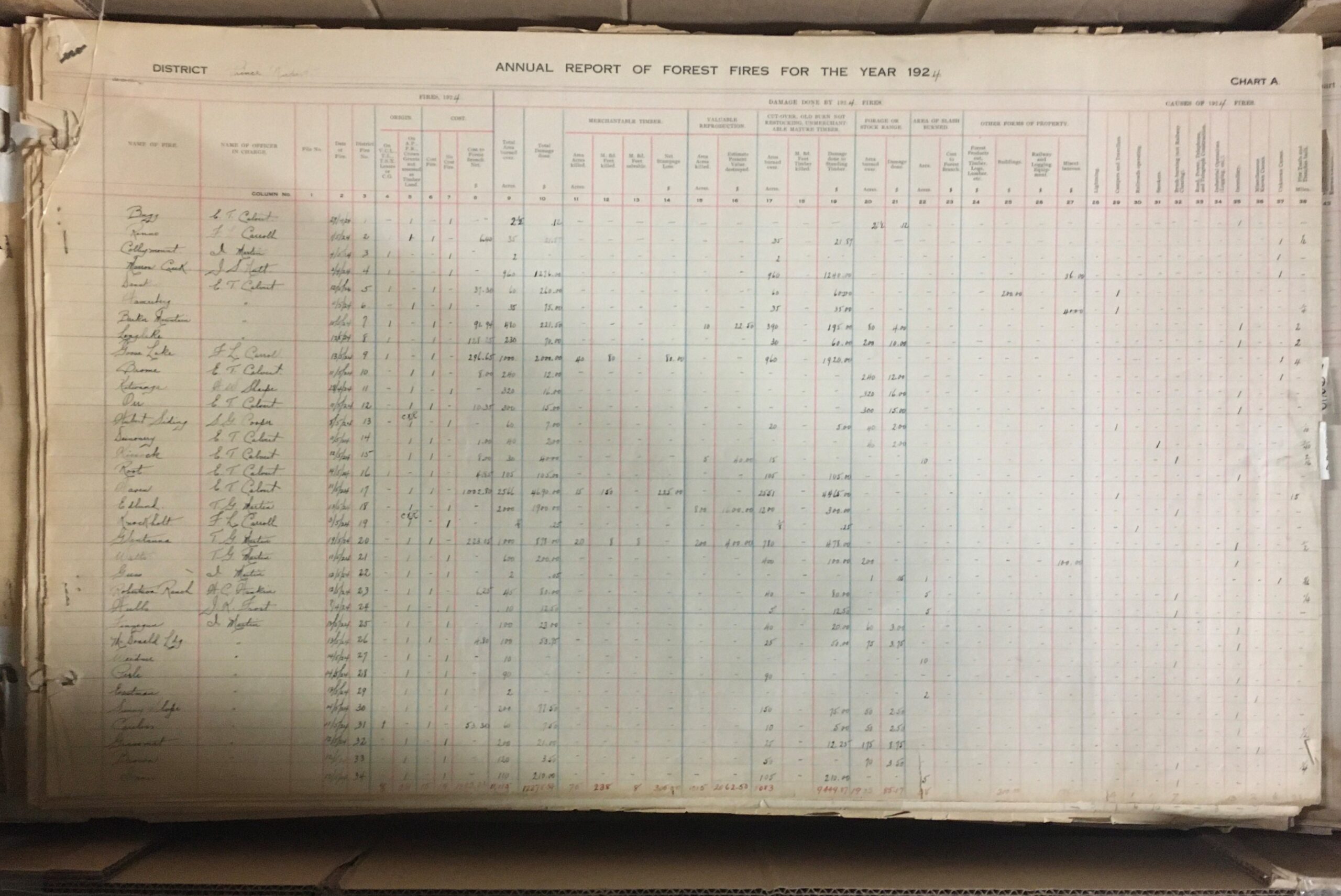 A page from the GR-4064 fire atlas. Each volume measures over three feet by four feet and weighed over 60 pounds.
A page from the GR-4064 fire atlas. Each volume measures over three feet by four feet and weighed over 60 pounds.
 A selection of volumes from GR-3929, including the first patient file from 1870.
A selection of volumes from GR-3929, including the first patient file from 1870.
Bonus records! Here are some other records that were made available a bit before 2021, but are still worthy of a mention:
*These series include sensitive personal information or other restricted records that are not publicly accessible.
 A public notice to be posted in schools, included with police records in GR-4000
A public notice to be posted in schools, included with police records in GR-4000
Lizard Lovin post
‘Tis the season: northern alligator lizards are busy making little lizards.
Have you seen alligator lizards mating? If so, please take note how long they spend together. Males bite the head and neck of females and hang on, sometimes for over 24 hours. Mating itself doesn’t take 24 hours—so perhaps males hang on to make sure other males are excluded.

In BC, the alligator lizards tend to vanish when new urban developments spread across the landscape—maybe (probably) the habitat is unsuitable, roadkill happens, a certain invasive lizard certainly doesn’t help, and well-fed domestic cats also take their toll, as do lawn mowers and weedwackers.

But if you are lucky enough to have northern alligator lizards in your garden, please take detailed notes on their activity, prey, and how they use your garden structures. It’s also helpful to record mating activity and how long a pair stays together. Community science (sometimes called citizen science) is a way to get very detailed views of species distributions to see how species are responding to our increasing sprawl.

Greg Pauly, curator of herpetology at the Natural History Museum of Los Angeles County, studies natural history, evolution and conservation of reptiles and amphibians, and the impact of urbanization on biodiversity.
You can share photos and information with Greg at gpauly@nhm.org.
Here are a few of his blog posts:
Love in the Time of Coronavirus: The Alligator Lizard Version
Look Out for Amorous Alligator Lizards
Studying Lizard Love Through Citizen Science
Alligator Lizards: Mating season has begun – YouTube
Government is constantly evolving to meet the needs of the citizens it serves. This often results in the renaming and reorganizing of its various branches and departments. These changes can make it tricky to track which government body was responsible for creating and maintaining particular records through the years. To help myself better understand who created some of the land records I was working with, I set out to create a visual representation of how the land management functions of government moved around over time. This soon expanded to include several other ministries whose records I worked on. It eventually grew to cover all ministries (and their precursor departments) from 1871, when the province was created, to 2021.
Please see BC Government Ministry History Diagram for the most current version of the diagram.
I created the chart using information from BC Archives authority records, Orders-in-Council and lists of Cabinet Appointments from the Legislative Library. Ministries are shown in square bubbles and are connected to each other with arrows. The oldest departments are at the top, moving down to the current ministries at the bottom of the page. The ministries are arranged in columns that represent broad categories of government functions, such as health or agriculture.
Arrows with solid lines show that a ministry was renamed. This usually means it continued to do pretty much the same work and fulfill the same functions of its predecessor.
Arrows with dotted lines show that some functions moved to another ministry. Dotted lines also show links from ministries that are established (created) and disestablished. I was only able to note large changes; sometimes the various branches that make up a ministry were split between a half dozen other ministries, making it pretty impossible to track all their movements individually.
The complexity and change over time make this a bit overwhelming to look at all together. But the changing names of a ministry can provide a high-level snapshot of general trends in government’s priorities over time. Each ministry is usually focused on a single function or issue, such as providing health care or managing public forests. The evolution of ministries reflects what government thought was important—and what mattered to voters. To a certain extent, this also reflects what mattered to society at large, though government was dominated by white men for most of the province’s existence, and universal enfranchisement did not occur until 1952.
Government departments changed very little from the province’s creation in 1871 until the 1970s. Government was small. Initially, the Attorney-General and Provincial Secretary were responsible for functions that would be divided among a dozen different ministers today. The major changes involved the creation of new departments as the role of government began to expand in the twentieth century.

Members of the Legislative Assembly demonstrating some pretty good social distancing. Photo taken in 1870 in front of the Victoria government buildings known as the Birdcages, after the union of the two colonies and just prior to Confederation. C-06178.
Initially, building infrastructure and promoting the industrial harvest of natural resources (to expand economic opportunities for other white settlers) appears to be more of a priority than providing social supports. For example, two separate departments for railways and public works were established almost 50 years before the Department of Health Services and Department of Social Welfare were finally created in 1959.
In 1976, all departments were renamed and became ministries. After this point, changes became much more common. By the 1990s it seems to have become fairly standard practice for a newly elected government to reorganize Cabinet, appointing new ministers to differentiate themselves from the previous government and reflect changing priorities throughout their term in office.
Some ministries have remained remarkably similar over the last hundred years. Others have been regularly broken up, combined and moved around in ways that, at times, can seem confusing and a bit counterintuitive. For example, in 1978, the Ministry of Recreation and Conservation was disestablished and broken into several ministries, including a new Ministry of Deregulation.

BC Legislative Assembly, Seventh Parliament, Third Session, in 1897 in the Birdcages. Construction of the current Legislative Assembly building was completed the following year. A-02563.
Names have power. Word choice can really impact how people perceive things, and the level of importance or value they place on an issue. The arm of government responsible for social services provides a good example of this. It has fulfilled fairly similar functions over time, but the language around the work has evolved alongside societal beliefs about poverty. It began in 1959 as the Department of Social Welfare. Later names included the Department of Rehabilitation and Social Improvement, the Ministry of Human Resources and the Ministry of Employment and Income Assistance. Currently, it is the Ministry of Social Development and Poverty Reduction.
Establishing (or disestablishing) a ministry shows that government is assigning its affairs a certain level of importance, with an elected official appointed to focus on the function or issue. The creation of a Ministry of Women’s Equality in 1991, when BC’s first female premier Rita Johnson was in power, shows women’s equality was an important issue for government at the time. In 2001, this ministry was disestablished and lumped into the Ministry of Community, Aboriginal and Women’s Services with four other disestablished ministries, including the also-disestablished Ministry of Aboriginal Affairs.
Overall, I have found the chart a helpful way to track government’s changing responsibilities at a glance. My hope is that it can be a useful tool for researching archival government records. A record’s creator (often the ministry or department that created it) is one of the main things archivists look at when arranging and determining different series of government records (GR-numbers) in the BC Archives database. If you can think about the ministry that may have created the records you are looking for, it may provide access to some unexpected results. You can search for archival records by creator here.
Happy researching!
Help shape teaching resources for your provincial museum.
The Royal BC Museum is looking for BC French teachers to participate in one of two focus groups in August. We are looking for teachers from across the K-12 spectrum to participate, (eight teachers in each focus group, ideally with a spread across grade ranges).
We want your input on the kinds of resources you’d like to have available from the museum. What would support you in the classroom, online or on field trips? You’ll be asked to assess what we have currently available, and then identify high priority gaps where you’d like to see resource development.
Compensation
Participants will be paid $100.
Focus Group Dates
Results from the focus groups will inform a secondary phase which will contract a French educator to develop the identified resources and make them available on the Royal BC Museum Learning Portal and ShareEdBC websites
Contact Liz Crocker lcrocker@royalbcmuseum.bc.ca to sign up.
As a government records archivist, I process a lot of records related to land or resource use, and specifically forestry records. Since the beginning of British Columbia’s colonization, forestry has been a major part of the province’s economy. After over 150 years of industrial logging, the majority of the province has been disturbed by some kind of industrial activity, leaving an estimated 2.7% as productive old growth forest today.
Most land in the province is Crown land controlled by the provincial government. Crown land and resources—in this case timber—are leased or licensed to forestry companies to use. These agreements are generally referred to as timber tenures and can include a variety of uses, lengths of time they are valid for and other required management practices.
Managing all these resources has created a huge amount of government records. Many of these are now kept by the BC Archives for government reference and historical knowledge, and so that the public can hold its government accountable. I recently processed a series of records related to forestry in the Clayoquot Sound area, now part of GR-3659.
GR-3659 documents a type of timber tenure referred to as a Tree Farm Licence (TFL). Most of western Vancouver Island is covered by a handful of TFLs. TFLs provide a forestry company with almost exclusive rights to harvest timber and manage forest in a certain area for 25 years. Every 5 years, companies are required to create detailed plans outlining how they will manage the land and resources within the TFL area.
Working with the records in GR-3659 got me thinking about a related historical event—the 1993 Clayoquot Sound protests, also known as the War in the Woods, which occurred primarily on TFL 54 and TFL 57. So, I took a look at some of the other records that document it.
Protests and blockades began in the 1980s after logging was proposed on Meares Island in Clayoquot Sound. Several attempts were made by the government to reach a consensus between the members of the forest industry, environmentalists, municipalities and Indigenous Peoples. These included the Clayoquot Sound Development Task Force (GR-3535, box 921203-0023) and the Clayoquot Sound Development Steering Committee. Environmentalists left the Steering Committee in 1991 after the government refused to defer logging until an agreement was made. The Steering Committee ultimately dissolved in October 1992, without completing a finalized development strategy.
A few months later, in April 1993, Cabinet passed the Clayoquot Sound Land Use Plan, but it was widely contested. The government’s lack of consultation with local First Nations and the large area of old growth forest available for clear cutting resulted in protests that spread around the world. Protest camps and blockades were set up to prevent logging, in defiance of court injunctions. Police attempts to enforce the injunction led to over 800 arrests. Thousands of people protested over the summer of 1993, making it possibly the largest event of civil disobedience in Canadian history.
Ultimately, the government was able to reach a consensus with the Nuu-chah-nulth First Nation, whose traditional territory includes Clayoquot Sound, and new land use plans were developed. This also led to the creation of Iisaak, a new First Nations owned logging company operating in the area (some of their records are in GR-3659), and the designation of Clayoquot Sound as a UNESCO Biosphere Reserve.

Letters protesting old growth logging received by the Premier in 1993 from across Canada and the rest of the world (BC Archives, GR-3571, box 921888-0036, file 16)

A small portion of the RBCM Native Plant Garden.
The native plant garden at the Royal BC Museum, with more than 300 species of plants, is a haven for wildlife in an otherwise heavily urbanized setting. The garden was initiated in 1968 in part to promote landscaping with our diverse native flora. The diversity of bird species that are known to visit the garden is truly impressive considering the downtown setting, and the garden also harbours a wealth of invertebrate animals: butterflies, bees, dragonflies, and damselflies, as well as many others.
Some of the animals are just passing through on their migration – for instance the eye-catching Green Darner dragonflies that have been seen there in the fall on their way south (yes! -some dragonflies migrate!) and the gorgeous Wilson’s Warblers that pass through on their way north each spring. Others are year-round residents in the garden: Song Sparrows, Spotted Towhees, while others come to the garden in the winter months and then head off to points north as the rest of the province warms up: Golden-crowned Sparrow, Ruby-crowned Kinglet.
It is not just that the garden offers a patch of greenspace though. What makes the garden a preferred location for our native wildlife are the native plants that inhabit it. The plant species that are native to this region co-evolved with the animals that also live here, seasonally or permanently – they need each other. There is no better example of this then examining the butterfly species reported from the garden. They come to this small area because the plant that they lay their eggs on and that their caterpillar eats can be found in the garden. Some examples include willows for two of our local Swallowtail butterfly species, Ocean Spray for the Lorquin’s Admiral, and Sedum for Moss’s Elfin. In the fall elegant Cedar Waxwings are regularly seen gobbling up the Black Hawthorn fruits, as well as those of some of the other shrubs in the landscape. These fruits are a critical food source for birds that they cannot typically find in urban settings.
The winter garden has value as well: native trees and shrubs offer a year-round source of food to the birds of the region because many invertebrates live on the plants; providing a source of protein to insectivores like Chestnut-backed Chickadees, Red-breasted Nuthatches, and Bushtits. This past winter a flock of as many as fifty Yellow-rumped Warblers spent many months foraging in the garden – always able to find food among the regionally-adapted plants because of the insects feeding on them.
Delta Surprise post
I don’t expect to get new lizard range records in mid-November, especially since the weather has been so grey and cool. This month it seems we have had wind storm after wind storm. Our northern alligator lizard (Elgaria coerulea) should be down for winter—maybe an occasional one will appear on really warm days. Common wall lizards (Podarcis muralis) will emerge, but only when it is sunny. I have seen wall lizards active in sun-exposed locations when the air temperatures are only 5–7°C. But since I have been indoors, I have resorted to correcting United States lizard misidentifications on iNaturalist.
This Monday morning (November 16th, 2020) is grey and cold, and another wind storm is due tonight. But to my surprise, I received an email from Nicole Greenbaum at Paridon Horticultural Ltd. in Delta, BC. She found a lizard in the company’s tropical greenhouse and had correctly identified it as a western fence lizard (Sceloporus occidentalis). Nicole’s discovery is western fence lizard #2 for British Columbia (and Canada).
Canada’s second confirmed western fence lizard; photo by Nicole Greenbaum.
The greenhouse is heated throughout the winter, and so if they are not able to catch this little lizard, it will have quite the winter. Of course I have asked them to catch it to represent BC’s first specimen for the museum collection. Photos are useful, but a specimen is better.
Who wouldn’t want to live in a tropical greenhouse? Photo by Nicole Greenbaum.
This lizard is a juvenile, with a body just over 3 cm. From the photos, I can’t tell whether it is male or female. But the record is now in iNaturalist.
It is possible that a female laid eggs and even more are in the greenhouse, but most likely, this is a single stowaway that came in with a shipment of plants. We have no idea where it originated, nor when it arrived. The greenhouse has not received plants from the United States in some time (it is 2020 after all), so the lizard has likely lived there all summer. I suppose that is effective, pesticide-free insect control.
I wonder when the next one will make its way north of the United States border?
A Tale for COVID Time
I have always believed that culture can transform, art can heal and history holds a mirror to ourselves and our time—thus my deep love for the study of history, arts and culture.
This current unsettling time of global pandemic crisis easily evokes feelings of insecurity and fear. It is a time of racism and uprisings against racism and other forms of discrimination. When I was given a small public space to share my work, I wondered what would be helpful when facing the challenges of our time.
The Royal BC Museum’s special Pocket Gallery A Tale of Two Families: Generations of Intercultural Communities and Family Lessons conveys a special message for the time of COVID. It assures us other people have not only made it through harsh times but have built legacies of success and community support.
Due to historical exclusion and colonial record-keeping practices, few families from settler minority groups can trace their histories back to the gold rush period that began in 1858. Since 2016, I have worked with two families that can: one French Canadian and the other Chinese Canadian. Their stories reveal rarely recorded generational continuities from the gold rush era to the present day. These families have survived times of great adversity, including the Great Depression and the Chinese exclusion era, to build lasting legacies in BC. What has kept the two families going for generations and through difficulties? What has allowed them to continue to grow and prosper today?
A deeper look at the family histories through the family members’ lenses reveals patterns in how the two families persisted through generations. Both families emphasize education, intercultural community building and kindness as family values. Their ideals resonate with our shared experiences and collective values as Canadians. During times of extraordinary challenges, such core values can shape people’s response. The stories of the Guichon and Louie families exemplify resilience through hard times in British Columbia history. Their family lessons are British Columbians’ strength.
On September 23, 2020, the museum hosted a special COVID-safe appreciation event for the featured families and communities of this Pocket Gallery. Maurice Guibord, président, Société historique francophone de la Colombie-Britannique, shared on video the significance of the Guichon family history.
Ms. Hilde Rose, the only living third-generation ranch operator, spoke on behalf of the Guichon family. With her husband Guy Rose, who was a third-generation Guichon, she operated the Quilchena Cattle Co. and the historic Quilchena Hotel for decades.
Mr. Alan Lowe, board chair of the Victoria Chinatown Museum Society and long-time beloved leader and former mayor of Victoria, spoke on behalf of the Victoria Chinatown communities to stress the importance of Chinatown history and the need to preserve it with a new museum in Victoria’s Chinatown.
Mr. Brandt Louie spoke on behalf of the Louie-Seto family as a direct descendant of the pioneer families of the Louies, Setos and Lees in BC, and of the Lews from the United States. He is also the third-generation leader of the H.Y. Louie Family Co. Ltd. His full speech can be found here.
The families’s joined commitment to education, intercultural community building and kindness resonate with our shared values as Canadians. These core values can help us shape our response during COVID time and the fight against discrimination through intercultural understanding and the pursuit of social justice.
Nature during COVID post
Royal BC Museum Biodiversity Research During the Pandemic
Nature is unrelenting. The zoonotic origin of the SARS-CoV-2 novel virus responsible for COVID-19 is a powerful reminder of the connections between human activities, health and biodiversity. As I write this, the COVID-19 pandemic continues to impact all aspects of human society, while the urgency to document, understand and protect biodiversity remains unabated. Museums in particular face the dual challenges of keeping the public engaged and maintaining their research programs. In many institutions, fieldwork, normally a major component of research activities during the summer months, has had to be postponed. Many aspects of learning, public engagement and curation of collections have moved online.
Researchers at the Royal BC Museum are considering the direct and indirect consequences of the pandemic on biodiversity while continuing to meet the challenges of biodiversity research. Aside from anecdotal reports (indicating, for example, that in many places biodiversity may be benefitting from reduced human activity), it is too early to provide a definitive answer to how the pandemic is affecting biodiversity. Given time, and with ongoing communication among scientists in natural history collections and the wider scientific community, this question can be answered by mobilizing and using biodiversity data found in natural history collections.
Ongoing communication of natural history collections is important, especially during this challenging time. Many people experience natural history collections only as museum exhibits. In this context, it can be difficult to visualize museum collections as baseline biodiversity data that are fundamental to diverse scientific fields. However, natural history specimens constitute the data for documenting biodiversity and constructing phylogenies, and they address evolutionary, ecological and biogeographic questions. Additionally, possibilities extend beyond the physical specimen, and may include genomic, behavioural and occurrence data. Therefore it is crucial that collections be maintained, that they grow, and that their data be continually enriched and shared. Through collections, we can improve our understanding of evolving species interactions, including those of pathogens and their hosts, which can help to better predict the risks of novel zoonotic diseases.
What about fieldwork? To cope with the travel restrictions and safety conditions surrounding the pandemic, some researchers have adopted different strategies. For example, in place of large-scale, rapid, people-intensive biodiversity surveys, some locations are studied in great detail over time, with fewer people. One project is the Quadra BioMarathon, conducted by researchers from the Hakai Institute to assess the diversity, abundance and composition of plankton just offshore from Hakai’s Quadra Island facility. The Royal BC Museum provides taxonomic expertise and advises on collection, preservation and identification of specimens.
Curators, collection managers and other researchers at the Royal BC Museum continue to work on collections, expanding, refining and making accessible the data through online collaborations with colleagues from as close as across town or as far as halfway across the globe. We are also committed to making biodiversity data and knowledge accessible to our diverse communities online.
Ongoing projects by Royal BC Museum researchers include taxonomic analysis, linking specimens to nucleotide, genome or protein data, and making data accessible through the Royal BC Museum online database.
Useful links:
GBIF Global Biodiversity Information Facility
iDigBio (Integrated Digitized Biocollections
Royal BC Museum Natural History Curators and Collection Managers
Dr. Victoria Arbour, Curator, Palaeontology
Dr. Henry Choong, Curator, Invertebrate Zoology
Dr. Joel Gibson, Curator, Entomology
Dr. Gavin Hanke, Curator, Vertebrate Zoology
Claudia Copley, Collections Manager and Researcher, Entomology
Heidi Gartner, Collections Manager and Researcher, Invertebrates
Piranha! post
“Don’t let it loose.” That is the message to all pet shops in the province. Shoppers should see the logo that the Inter-Ministry Invasive Species Working Group (IMISWG) asked pet shops to display:

But clearly this message is not getting through.
A blue-eyed panaque was caught in a ditch leading to Shawnigan Lake.
A pacu was caught in Green Lake, Nanaimo.
An albino oscar was caught in Chemainus.
A flowerhorn cichlid was found in Kelowna. Flowerhorns are a man-made hybrid and do not exist in the wild.
Goldfish and rosy-red (fathead) minnows appear in BC with alarming regularity.
All of these abandoned animals originated in the pet trade.
And in 2019, two redbelly piranhas (above, the caught in September; below, the one caught in July) were found in Westwood Lake in Nanaimo. This is a South American species with a famous reputation, exaggerated by several really bad B-movies.

Piranha also may have been released in Langford Lake—a fish enthusiast who routinely dumped his piranha when they grew too large was reported by a friend. We are fortunate that piranhas won’t survive our winters and that most BC lakes are too cold to sustain them even in summer. However, it does show the frequency with which pets get dumped, and you have to wonder what motivates people to abandon pets, especially pets that are potentially harmful to the environment (and in this case, to people).
According to Fuller et al. (1999), the native range of the redbelly piranha is lowland central and southern South America east of the Andes, including the Amazon and Parana basins and the coastal drainages of Brazil and Guianas. In North America, the species has been found in Florida, Hawai’i, Massachusetts, Michigan, Minnesota, Ohio, Oklahoma, Pennsylvania, Texas and Virginia. You can bet all of these are aquarium releases. Individual white piranha (S. rhombeus) also have been released in Florida, and a sinkhole pool at a Florida tourist attraction was stocked intentionally in the 1960s. The pool supported a population for about 14 years before state officials used rotenone to wipe them out. Other piranha, which were not identified, have appeared in California, Connecticut, Florida, Idaho, Illinois, Iowa, Kentucky, Missouri, New Hampshire, New York, Oklahoma, Pennsylvania, Utah and Washington.
I don’t think we have a synopsis of records in Canada, but I know pacu and silver dollars—relatives of the piranha—have appeared in Manitoba and Ontario, so who knows how many piranha have also been dumped in this country.
If you don’t want a fish, take it back to a pet shop, find it a new home, or if you have to, have it euthanized humanely. Our waterways are cool to cold, and it is unfair to make tropical fish suffer in cold water. And released tropical fish present a disease risk to our native fishes. Don’t release pets. BC becomes less beautiful with every abandoned animal (and aquarium plant—but that is another story entirely).
Reference:
This is the month that the garden explodes with food—and lizards. Everywhere you look, there is food to harvest. We try to weigh everything to get an idea of the cash value in our garden, but sometimes you just have to eat things right off the vine.
Our tomatoes are ripening daily, with the Pineapple Cherry tomatoes at their delicious best now that we are nearing the end of August. Several of our tomatoes are not your typical variety: the Snow White ripens a pale cream-white colour, Black Prince are red-brown when ripe, and then we have the Green Zebra, which (as the name suggests) is striped green when ripe. They are all fantastic.
We have already started saving seeds for next year. The seeds are left in water for a few days to simulate the rotting fruit, then we drain and dry to store seeds over winter.
Zucchini, cucumbers, squash and melons are all producing fruit, though I don’t think the melons will amount to anything. Our pumpkin went nowhere. C’est la vie dans la grande ville. We will buy pumpkins to carve for Halloween and support a local grower in the process.
Cabbages are being converted to sauerkraut. Some cucumbers were grown specifically for pickles, others to eat straight up.
We have collected over 6 kg of blackberries, and our strawberries are still producing. The yellow raspberries produced just enough fruit for all three of us to enjoy a quick snack.
Apples are nearly ready. And if this wasn’t enough fruit, our neighbours have offered some pears—we just have to pop over and pick them.
A grey squirrel has figured out that our garden has loads of food and destroyed our mini-sunflowers. It also has been eating plums, leaving sticky scraps on our compost bins. That is part of gardening—sometimes you have to share. Wasps and house finches have nibbled on their fair share of the blackberries.
Even without our greenhouse—it got crushed by the snow last winter—we still grew some nice peppers, and the Italian parsley and New Zealand spinach are growing well. Carrots are our biggest failure; only a few grew.
Lizards have hatched—everywhere you look, a lizard the size of my pinkie finger darts off to hide in our rock walls and garden beds. The adults are still here too—but they seem to be less interested in basking in the midsummer’s sun. Perhaps the females don’t need to bask so much now since they are not carrying developing eggs.
Much of the work now shifts indoors. Berries and tomatoes are being frozen for winter use. Beans are being shelled and left to dry for winter chili, stews and soup. Onions are pulled and drying on the deck so they will store well. Only the Scarlet Runner Beans are still growing and will be picked later in the season.
The bean plants that are finished are now pulled and left on the ground to decay and add substance to the soil. We still need to harvest the potatoes in our front garden, and then in the next few weeks we will plant a cover crop. Once the cover crop has grown a few centimetres, we will cover it with a tarp to let worms and other recyclers enrich the soil.
And even while the harvest is in full swing, we are planting lettuce and kale starts for autumn and winter. It is amazing to be able to walk outside in winter and get fresh produce when you need it.
This has been a good summer for food production. We have hundreds of dollars in produce, with over $50 in blackberries alone at current market rates. We added oak-leaf mulch and some horse manure early in the season, watered when we needed to, and that is about it. Even a modest city garden can produce plenty of good clean food.
Hatchlings Happen post
I have been watching a female lizard in my garden slowly increase in girth as her eggs developed in May to early June. I recorded each day whether she was gravid or not, and noted when she lost her tail. Simple observational science is something I could do to better understand common wall lizard biology in BC while working from home.
My notes around the time I think eggs were laid went as follows:
June 5 – basking on rocks, 80% tail lost in the last few days, still looks gravid.
June 6 – not sighted
June 7 – not sighted
June 8 – not sighted
June 9 – rainy, cold, not sighted
June 10 – basking on rocks, has folds of skin along the flank suggesting eggs laid.
I have watched our resident female wall lizard as her tail regenerated, and I saw her again yesterday (July 30) sporting a tail several centimeters shorter than the original.
I patrol the garden daily to see what the lizards are doing, and when and where they are active. My wife will confirm this obsessive behaviour. But this morning (July 31) I dropped my daughter off at a day camp, and on return home, as I walked up my driveway, I spotted our first hatchling.
I gasped—I am not ashamed to admit it.
Lizards invaded our garden in 2019, and one year later we have a home-grown baby lizard. It looked pretty comfortable in its new habitat, so perhaps it hatched yesterday (July 30) or the day before. And there is no way to tell, without perhaps a series of DNA samples, whether this hatchling came from the female I have been studying, or whether some other female dug a nest in our garden. Young lizards leave their parents’ territory to avoid cannibalism, so this one may head for the hills. But it does give us an estimate of timing between egg deposition (somewhere between June 6–8, assuming eggs were not laid June 9 when it was cool and rainy) and the first appearance of hatchlings July 31.
Females mature in their second year and can have one to three clutches of eggs each summer, depending on latitude and local conditions, with clutches ranging from 2 to 10 eggs. In nature, incubation ranges from 6–11 weeks. Embryo development is about halfway at oviposition, and females bury eggs in sandy or crumbly substrates at the end of 10–20 cm tunnels (Van Damme et al. 1992). In a laboratory experiment, temperature dramatically changed incubation times, with wall lizards incubated at 32–35°C hatching 10 days earlier than lizards incubated at 28°C, and over five weeks earlier than those incubated at 24°C (Van Damme et al. 1992). We had a cool spring, and I am not surprised then that 50–52 days passed between the first evidence that the female had laid eggs and the appearance of a hatchling.
Hatching success is high in the 24–28°C incubation range, and Van Damme et al. (1992) suggested 28°C is the best trade-off between hatching success, incubation rate and hatchling health. I wonder how many more hatchlings will appear in the garden?
For more information:
Van Damme, R., D. Bauwens, F. Braña and R.F. Verhyen. “Incubation Temperature Differentially Affects Hatching Time, Egg Survival and Hatchling Performance in the Lizard Podarcis muralis.” Herpetologica 48, no. 2 (1992): 220–228.
Ooooohhh Barracuda post
It is 2020, and people are asking whether this year will get any stranger.
How about barracuda in BC waters? Does that qualify?
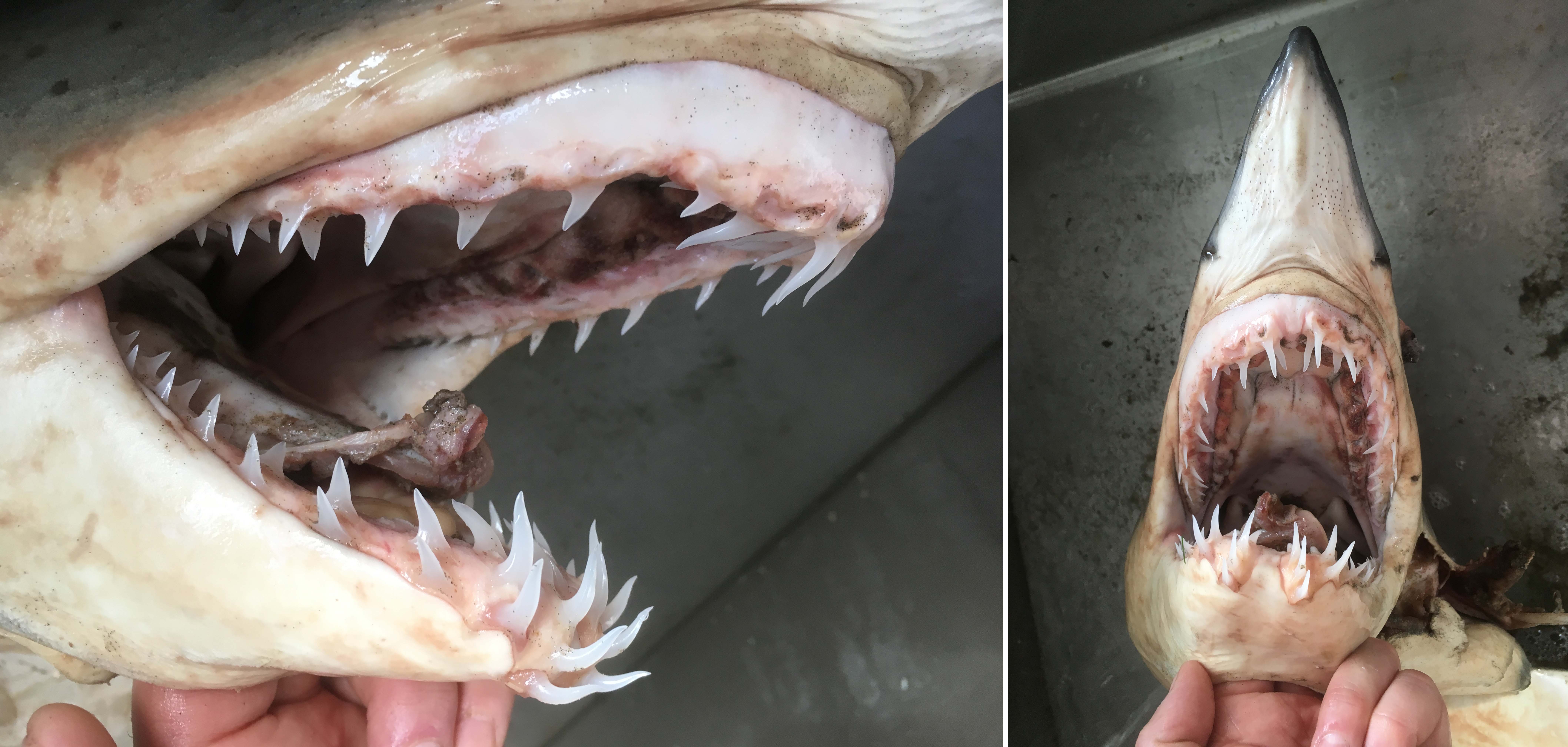
I received several emails and other messages today (July 10) noting that a 5.4 kg barracuda had been caught off Vancouver Island this week. This is a really cool record, and I hope it’s added to iNaturalist.

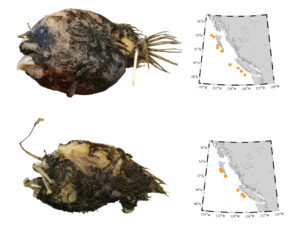
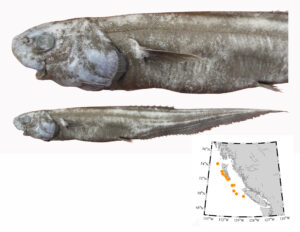
Pacific barracuda (Sphyraena argentea) from San Diego, California. Photo by Darren Baker, uploaded to Fishbase IMG-20120830-00071.jpg.
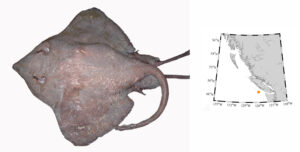
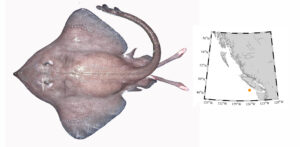
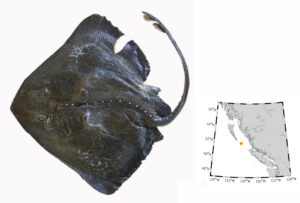
Pacific barracuda (Sphyraena argentea) are known to range all along our coast, and as Alaska-based biologist Scott Meyer notes, they range north to Alaska during El Niño years. The first record from Alaska (off Kodiak Island) dates back to 1937, when a school of barracuda was sighted, though only one was caught. The surface waters of the Eastern Pacific Ocean must have been warm that year, because a barracuda also was caught off Sooke, British Columbia. Another barracuda was found in Prince William Sound, Alaska, in 1958. In BC they are known also from Queen Charlotte Sound and the Prince Rupert area (see Hart 1973). Pietsch and Orr (2019) detail several barracuda records from the Salish Sea in their magnum opus, Fishes of the Salish Sea.
The first record along the BC coast was a specimen cataloged at the Royal BC Museum (RBCM 33), caught at Otter Point in Sooke, July 27, 1904. It is the only Pacific barracuda in the RBCM collection. According to Peitsch and Orr (2019) the earliest record of Pacific barracuda in the area comes from Gig Harbor, Puget Sound dating back to 1878.
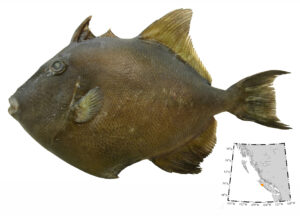
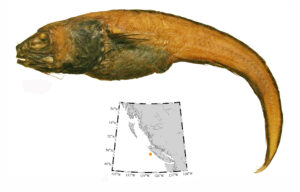
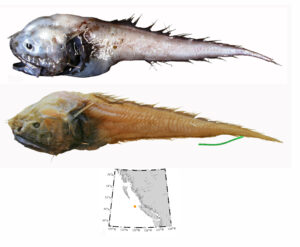
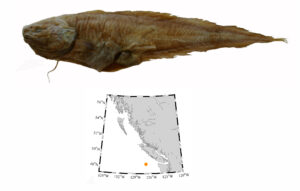
I wouldn’t mind another specimen for the museum collection to accompany the 1904 specimen and our other warm-water strays: the louvar and finescale triggerfish found here in 2014, the North Pacific argentine from 2010 and the spotted porcupinefish from 2019.
I wonder what fish is next? Maybe we will get more hammerhead sharks? They were seen off Ucluelet in 1952 and 1953. Sure would be neat to have them here again.
References:
Carl, Clifford C. “The Hammerhead Shark in British Columbia.” Victoria Naturalist 11, no. 4 (1954): 37.
Cowan, Ian McTaggart. “Some Fish Records From the Coast of British Columbia.” Copeia 1938, no. 2: 97.
Hart, John Lawson. Pacific Fishes of Canada. Fisheries Research Board of Canada Bulletin 180. 740 p.
Quast, Jay C. 1964. “Occurrence of the Pacific Bonito in Coastal Alaska Waters.” Copeia 1964, no. 2: 448.
Pietsch, Theodore, and James. W. Orr. Fishes of the Salish Sea, Puget Sound and the Straits of Georgia and Juan de Fuca. Victoria: Heritage House, 2019. 1032 pages.
Van Cleve, Richard, and W.F. Thompson. “A Record of the Pomfret and Barracuda from Alaska.” Copeia 1938, no. 1: 45-46.
Authors: Robert J.WilliamsaAlison M.DunnaGavinHankebJoel W.DixonaChristopherHassalla
ABSTRACT
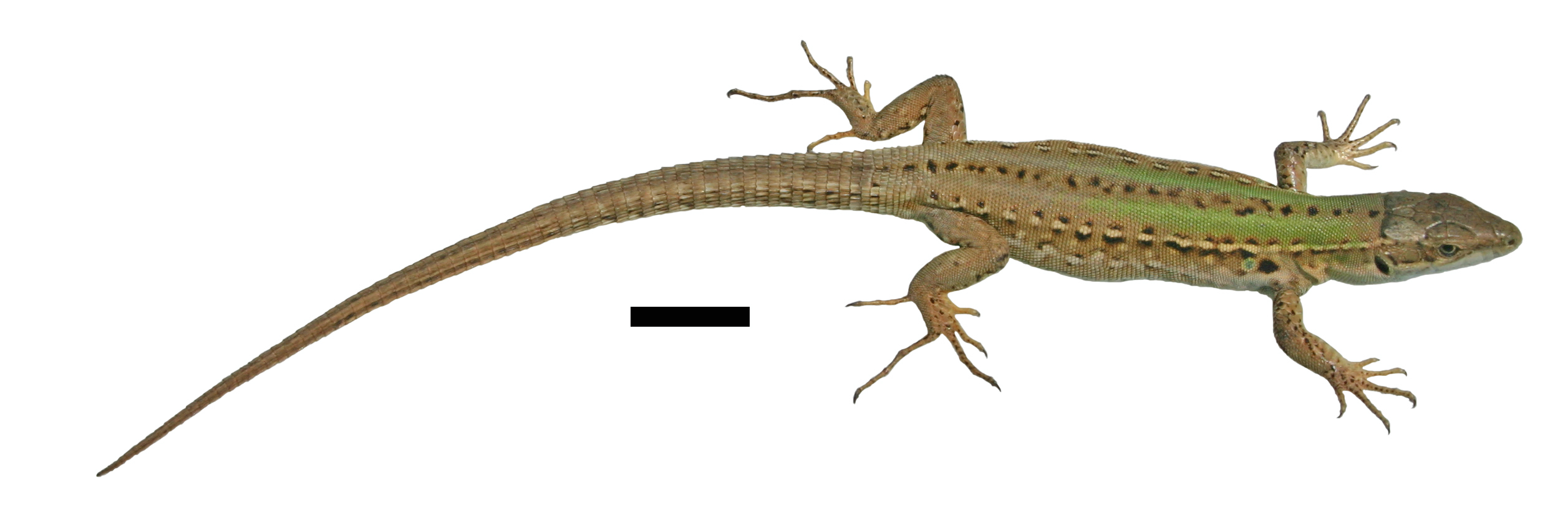
The human-assisted movement of species beyond their native range facilitates novel interactions between invaders and native species that can determine whether an introduced species becomes invasive and the nature of any consequences for native communities. Avoiding costly interactions through recognition and avoidance can be compromised by the naïvety of native species to novel invaders and vice versa. We tested this hypothesis using the common wall lizard, Podarcis muralis, and the native lizard species with which it may now interact in Britain (common lizard, Zootoca vivipara, sand lizard, Lacerta agilis) and on Vancouver Island (northern alligator lizard, Elgaria coerulea) by exploring species’ responses (tongue flicks, avoidance behaviour) to heterospecific scent cues in controlled experiments. The tongue flick response of P. muralis depended on the different species’ scent, with significantly more tongue flicks directed to E. coerulea scent than the other species and the control. This recognition did not result in any other behavioural response in P. muralis (i.e. attraction, aggression, avoidance). Lacerta agilis showed a strong recognition response to P. muralis scent, with more tongue flicks occurring close to the treatment stimuli than the control and aggressive behaviour directed towards the scent source. Conversely, Z. vivipara spent less time near P. muralis scent cues than the control but its tongue flick rate was higher towards this scent in this reduced time, consistent with an avoidance response. There was no evidence of E. coerulea recognition of P. muralis scent in terms of tongue flicks or time spent near the stimuli, although the native species did show a preference for P. muralis-scented refuges. Our results suggest a variable response of native species to the scent of P. muralis, from an avoidance response by Z. vivipara that mirrors patterns of exclusion observed in the field to direct aggression observed in L. agilis and an ambiguous reaction from E. coerulea which may reflect a diminished response to a cue with a low associated cost. These results have significant implications for the invasive success and potential impacts of introduced P. muralis populations on native lizards.
Keywords
See full article
See Claudia’s Research Gate profile, which includes her recent publications and peer reviewed articles.
Abstract
See full article
This article examines the Punjabi Canadian Legacy Project (PCLP), a partnership project between the Royal British Columbia Museum and the South Asian Studies Institute at the University of the Fraser Valley, as a case study of heritage from below. The project is based on community action research and practices, joining forces of memory, research, and community institutions, organisations, and groups. Considering ‘heritagisation’ as a process of heritage building, and drawing on their experience as practitioners on this project, the authors argue for the need to consider the vast diversities within and among communities, and the need to work on ‘heritagisation’ through ongoing dialogic engagement.
Through a myriad of continuous dialogues and inherent challenges, the process and progress of the PCLP is shaped by this dialogic engagement. As an ongoing project, the PCLP demonstrates how a network extended to diverse participants with shared goals can emerge through the organically developed heritagisation process encouraged by the partners’ collaborative efforts in an experimental model for community work by memory and research institutions.
See full article
A Growing Tale post
Working at home has allowed me to pay plenty of attention to the individual lizards in our garden. I can watch where they go, locate preferred basking spots in the garden, watch what they eat, and try to figure out when eggs have been deposited and, later, when hatchlings will emerge.
Individuals are easy to identify based on size, sex and scarring. Most lizards have tails that have regrown, and the relative length of the original tail helps identify each animal.
Our gravid female had a perfect tail until recently, but on June 5, I noticed that she had been attacked. Her tail was now less than a quarter of its original length. The stump was still fresh and had not grown over with new skin.
We have several domestic cats vying for our garden (they also like using our garden beds as a litter box). The complexity of our garden attracts lots of birds, and it’s prime hunting territory for pudgy suburban felids to roam and kill at will. One cat (we know him as Meow, because that is what he said when we asked him his name), is a frequent visitor to our yard. He is likely the local lizard lacerator.
It appears that the growth is slow at first as the tail heals and the tissues organize themselves, but between June 19 and July 4, the tail grew an estimated 26 mm. The regrown tail will never be as long as the original, but it can be shed again if the lizard is attacked.
Nature is amazing. Field bindweed, an invader from Eurasia, grows at an alarming rate in our garden. Beans can go from a mere sprout to a massive flowering plant in a few weeks. And we can add lizard tails to our list of fast-growing crops.
When we harvest leafy veggies like lettuce, chard or New Zealand spinach, we take a few leaves for our meal and leave the rest of the plant to grow. The would-be predator attacking the wall lizards in our yard is also harvesting tails and letting the lizards regrow a new crop. I don’t think domestic cats have the mental capacity to intentionally farm lizard tails, but that is effectively what is happening.
Robb Bennett¹,², David Blades², Gergin Blagoev³, Don Buckle⁴, Claudia Copley², Darren Copley², Charles Dondale⁵, and Rick C. West⁶
1 Corresponding author – robb.bennett@shaw.ca
2 Natural History Section, Royal British Columbia Museum, 675 Belleville St, Victoria, BC, Canada
3 Centre for Biodiversity Genomics, University of Guelph, Guelph, ON, Canada
4 16-3415 Calder Crescent, Saskatoon, SK, Canada
5 Canadian National Collection, Agriculture & Agri-Food Canada, 960 Carling Ave, Ottawa, ON, Canada (retired)
6 6365 Willowpark Way, Sooke, BC, Canada
Abstract:
In 2006, the Royal British Columbia Museum began systematically documenting the full diversity of British Columbia’s spider fauna. Initially, museum specimens and literature records were used to update an existing checklist and identify poorly sampled habitats in BC. Annual field surveys of spiders, primarily targeting alpine and subalpine habitats, began in 2008; barcode identification of previously unidentifiable specimens commenced in 2012. These efforts have resulted in significant increases in the area of BC that has been sampled for spiders, the number of species documented in the BC checklist, and the number of specimens in the RBCM collection. Many of the additions to the checklist represent the first Canadian or Nearctic records of those taxa or are undescribed species. By 2017, data from more than 9000 spider specimens had been entered into the RBCM database.Data from many specimens, however, remain unrecorded and currently (2017) the RBCM collection is estimated to house more than 90 000 specimens. The number of species recorded in BC has climbed from 212 in 1967 through 653 in 2006 to 859 in 2017. Here we present BC localities data and general global distributions for those 859 taxa. The progress of the RBCM’s work has made the RBCM an important repository of western Nearctic spiders and shown that British Columbia is an important area of Nearctic spider diversity.
See full article
ABSTRACT
The distribution of northern British Columbia alpine plants is poorly documented. To improve our understanding of the flora of this vast, remote region, we collected more than 11 000 specimens from 65 mountains during 2002–2011. Most of these locations had not been visited by botanists. Of the more than 400 species we have collected, two are new to the province, others represent significant range extensions. Twelve species share elements of a disjunct distribution that has apparently not been previously recognized and consists of three regions: (1) northwestern North America; (2) Beartooth Plateau; and (3) northern Colorado. These 12 species appear to be absent from the extensive areas of suitable habitat that occur in the intervening areas. The most reasonable explanation for this pattern is that these species, adapted to arctic–alpine tundra conditions, migrated throughout western North America during the Pleistocene, a time when suitable habitat was much more widespread than now, and subsequently went extinct in many areas as the climate warmed during the Holocene.
Keywords: arctic–alpine vascular plants, disjunct, northwestern North America, Beartooth Plateau, northern Colorado
See full article
Abstract
Many plant species comprising the present-day Arctic flora are thought to have originated in the high mountains of North America and Eurasia, migrated northwards as global temperatures fell during the late Tertiary period, and thereafter attained a circumarctic distribution. However, supporting evidence for this hypothesis that provides a temporal framework for the origin, spread and initial attainment of a circumarctic distribution by an arctic plant is currently lacking. Here we examined the origin and initial formation of a circumarctic distribution of the arctic mountain sorrel (Oxyria digyna) by conducting a phylogeographic analysis of plastid and nuclear gene DNA variation. We provide evidence for an origin of this species in the Qinghai-Tibet Plateau of southwestern China, followed by migration into Russia c. 11 million yr ago (Ma), eastwards into North America by c. 4 Ma, and westwards into Western Europe by c. 1.96 Ma. Thereafter, the species attained a circumarctic distribution by colonizing Greenland from both sides of the Atlantic Ocean. Following the arrival of the species in North America and Europe, population sizes appear to have increased and then stabilized there over the last 1 million yr. However, in Greenland a marked reduction followed by an expansion in population size is indicated to have occurred during the Pleistocene.
Keywords: ancestral location; arctic flora; circumarctic distribution; migration; species origin.
See full article
Abstract
Aim
Many plants, especially at high latitudes, have both widespread and highly discontinuous geographical distributions. To increase understanding of how such patterns originate, we examine genetic patterns in the arctic–alpine plant Sibbaldia procumbens . We evaluate the contributions of refugia and the role of long‐distance dispersal in shaping the current range of this species.
Location
Northern Hemisphere, especially North America.
Methods
We sampled Sibbaldia from 176 localities, including 168 for S. pro‐cumbens . We analysed sequence variation in three plastid DNA non‐coding regions (the atp I–atp H and trn L–trn F intergenic spacers and the trn L intron), performed Bayesian phylogenetic analyses and statistical parsimony analyses on the combined sequences, and analysed the geographical patterns of haplotype distribution and genetic diversity using data from all populations.
Results
Sibbaldia procumbens probably originated in the mountains of South and East Asia. We identified highly distinct clades in Europe and North America, which overlapped on oceanic islands of the North Atlantic indicating long‐distance dispersal capability. The North American clade included two lineages, one in California and the other widely distributed across the continent and North Atlantic. Haplotype diversity in the latter lineage was markedly higher to the south, suggesting mid–late Pleistocene southward displacement of North American populations with subsequent migration northwards into previously glaciated regions. In Europe, disjunct geographical regions generally harboured distinct haplotypes.
Main conclusions
Multiple Pleistocene refugia for S. procumbens occurred in both North America and Europe. North American refugia existed in California and in the southern Rocky Mountains, but in contrast with most widespread arctic–alpine species we found no evidence for a Beringian refugium. Cryptic refugia may have existed within the Cordilleran Ice Sheet. Episodes of range expansion and contraction and long‐distance dispersal have all contributed to the genetic structure and widespread but fragmented distribution of this species.
See Full Article
I recently spent a glorious sunny day on Willows Beach in Victoria. Staghorn sculpins (Leptocottus armatus) and many small soles raced to deeper water as we walked along in ankle-deep water. The tide was out. People were everywhere, but no one was lifting rocks to see everything hiding in plain sight.
Further up the beach was a line of marine macroalgae (especially Ulva, sea lettuce) stranded by the receding tide. Under each rock you can expect a handful of isopods, as well as shore crabs and small Dungeness crabs that scuttle away, and even the tiniest puddles under a rock sheltered up to 8 fish—sculpins and gunnels. The sun heated the beach, but under the algae-draped rock, water stayed shaded and cool and kept everything alive until the tide returned.
Most pools had tidepool sculpins (Oligocottus maculosus), and most were the typical grey-brown mottled form. But there also were many of these green sculpins—the same species as the typical grey-brown tidepool sculpin. These green sculpins are perfectly camouflaged in patches of sea lettuce.
A bright-green tidepool sculpin sure stands out from its typical grey-brown relatives.
Without the usual coloration to rely on, you have to look more carefully to see whether this is a tidepool or fluffy sculpin (O. snyderi). Fluffy sculpins have cirri (little wispy flaps of skin) along the lateral line in clusters of three to six, and the clusters of cirri follow the lateral line along the flank of the body. This photo—even though it was taken with my old iPhone 6. which performs poorly when taking macro shots—shows that the cirri along the lateral line are only found near the head, and they are single. This clearly is not a fluffy sculpin.
Next time you are on the beach and the tide is out, take some time to explore tidepools and rocky ledges along the shore, and carefully lift some rocks. You probably will find a lime-green sculpin or two. You may also find lime-green penpoint gunnels (Apodichthys flavidus) or rosylip sculpin (Ascelichthys rhodorus). If you are really lucky, you will find sculpins with bright-pink colouration to match coraline algae, or a blue-and-red-banded longfin sculpin (Jordania zonope). Even in this city, there is plenty to see along shore.
Don’t Fence Me In post
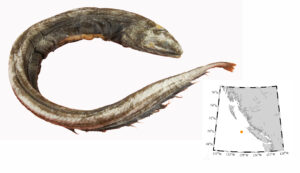
On June 16, I received notification that my blog post on introduced lizards in Hawaii was live on the internet. The last paragraph in that article is about western fence lizards (Sceloporus occidentalis), where I ask people who see them in BC, to report the observation (with a picture if possible), or tag the occurrence in iNaturalist. Western fence lizards were on Matsuda et al.’s (2006) radar as a potential immigrant since the species exists so close to the Canadian border.
Between submitting that blog post and it going live, the plea for lizard sightings was answered. A report came my way of a western fence lizard loose in BC. I had always assumed the first record would surface in the Okanagan region, since several anecdotes from there suggest fence lizards are already present as far north as Oliver. Furthermore, field guides show western fence lizards just south of the Okanagan region (St. John 2002; Stebbins and McGinnis 2018) and Storm et al. (1995) presented a range map for western fence lizards having a straight line at the international border. Lizards don’t recognize political boundaries, so there is no way Storm et al.’s map is accurate. Fence lizards would do really well in the orchards, fence lines and piles of fruit crates of the southern Okanagan.
Instead of the Okanagan, the first record of a western fence lizard in British Columbia came from the Cloverdale area of Surrey, on June 6, 2020.
Our first confirmed western fence lizard was a juvenile, and it had lost its tail to some would-be predator. We have no idea how this lizard arrived in BC; most likely it is a stow-away from south of the international border. It could have been an escaped pet, or maybe there is a population now in the area that has gone unreported. The lizard is still loose, but we are hoping to catch it and add it to the museum collection.
The lizard is strangely coloured for a western fence lizard, but it does have the yellow-orange tint to the rear surfaces of the fore and hind limbs. The other possible look-alike in the region, though not in Canada, is the sagebrush lizard (Sceloporus graciosus), which has white on the undersides of its limbs. Sagebrush lizards are in Washington, but nowhere near as close to BC as western fence lizards (Storm et al. 1995; St. John 2002; Stebbins and McGinnis 2018). The nearest population of western fence lizards in Puget Sound is at Cherry Point, about 27 km south of where the Cloverdale specimen surfaced, thanks to a researcher in 1990 who released a handful of fence lizards to see if a population would get established.
In addition to this Cloverdale record in Surrey, a western fence lizard was reported on iNaturalist, April 2019, at MacNeill Secondary School, Richmond, British Columbia. However, this 2019 report cannot be verified since neither specimen nor photograph are available. Perhaps a second western fence lizard is loose in BC. Maybe it’s a sagebrush lizard. It would be great to get a picture of that Richmond reptile to verify the species.
And I am still not giving up on the Okanagan—if you live anywhere between Summerland and the international border, keep your eyes peeled for fence lizards!
Some background information:
Matsuda, B. M., D. M. Green, and P. T. Gregory. 2006. Amphibians and Reptiles of British Columbia. Royal BC Museum, Victoria, British Columbia, Canada.
Stebbins, R. C., and S. M. McGinnis. 2018. Peterson Field Guide to Western Reptiles and Amphibians. Houghton Mifflin Harcourt, Boston, Massachusetts.
St. John, A. 2002. Reptiles of the Northwest, British Columbia to California. Lone Pine Press, Renton, Washington.
Storm, R. M., W. P. Leonard, H. A. Brown, R. B. Bury, D. M. Darda, L. V. Diller, and C. R. Peterson. 1995. Reptiles of Washington and Oregon. Seattle Audubon Society, Seattle, Washington.
Rain this June has reduced our watering bill. Rain helps the plants we want, and also helps the weeds, so we spend a few hours each weekend weeding here and there. We are now well into the growing season, and we got a few new plants. From left to right below: the wasabi plant; behind it, an acacia (which will be potted—can’t wait to see it flower); a raspberry that creeps along the ground (YAY—ground cover in the food forest that produces food and limits the regrowth of grass); and a gooseberry. The ferns (below) we have had for a while in the shade along the side of the house and they need some TLC. Both sides of the house need some thought and effort once the main food-producing areas are established. The wasabi is growing well, and that acacia was so crazy looking that I couldn’t resist.
In the front food forest we have apples, cherries and blueberries well on the way.
Beans, onions and potatoes are growing fast.
The honey berries ripened early and we had them in pancakes. The front third of the garden is covered in wood chip and has three new sea buckthorns (far right below), a rose (middle) has set itself along the driveway, and I buried a few salmon berries to see if we can grow a few of those—that’ll bring in the hummingbirds. It is an area the deer can access. They seem to leave it all alone, and now that the grass is bashed back, only minor weeding is needed.
The back yard is looking lush. Along the eastern fence (on the right in this panorama) is our thornless blackberry—last year we had about 3 kg of berries—this year it is COVERED with flowers. What a bounty—and we save them frozen for winter crisps and smoothies (or for a small human who likes eating frozen berries like candy). The area around the sunken patio needs some effort—there are herbs in there—but the grass and other agressive plants have run wild.
The peppers, squash, cucumbers and pumpkin are growing nicely out back, although they are along the west side of the garden and our neighbour’s cedar hedge does drain the life from that soil. Plants grow poorly the closer they are to the cedars. Volunteer kale holds on and will seed again—yay kale.
Strawberries are ripening daily. You can’t beat the flavour of home grown strawberries warmed in the sun.
Ladybird beetles are everywhere. We have at least seven lizards in the front garden, and maybe three or four in the back. The female wall lizard which was obviously loaded with eggs now looks lighter, so we can expect hatchlings in a few weeks. The photo below is the male that may be the father of this year’s hatchlings, and as far as I can tell, our only garden lizard in the front yard with an intact tail.
The poppies are popping up all over the place. Tomatoes and cabbage are rocketing up, and the New Zealand spinach is ready for selective harvest. Chives have gone to seed, a leek has a seed head taller than Anna, and the lettuce is still growing well. There’s so much to eat in such a small space.
The usual avian suspects are flitting about—Bewick’s wrens; crows, which are now dive-bombing pedestrians; robins, which have nested and are much quieter; and Anna’s hummingbirds, chipping sparrows and house finches, which pop by regularly. We were buzzed by an osprey the other day—that was neat to see.
And I finally got around to protecting our grape with netting (above). The local mule deer seem to notice it once it grows about six leaves, and then they strip the plant. Not this year.
The garden is never finished, and it is always a learning experience. We are starting a chop-and-drop program to leave cut debris in place rather than taking it over to a separate compost pile. The chopped and dropped debris acts like leaves in a forest to reduce rain compaction of soil, and eventually it rots and adds organic matter to the surface – to be re-worked by nature. Probably the greatest thing to see is our daughter learning about the seasons in the garden, where food really comes from, what is ready to eat and what is not.
Cork It post
What does a cork have to do with my research? Cork was produced in Europe and North Africa and shipped to North America in bulk until the Atlantic was blockaded during WWII. Stow-away pests were inevitable.
In the 1940s, the western green lizard (Lacerta bilineata) was introduced repeatedly to Gloucester, New Jersey, as a stowaway in bales of cork bark (Kraus 2009; Burke and Deichsel 2008; Lever 2003; Conant 1945). In 1944, a single specimen of the ocellated lizard (Timon lepidus) also was caught on the Gloucester piers (Conant 1945). Common wall lizards (Podarcis muralis) also appeared over several years in New Jersey in the 1940s from shipments of cork bark (Kraus 2009; Conant 1945). The diversity of stowaway lizards underlines the ease and risk of accidental transport of lizards. Maybe the switch to synthetic stoppers has plugged this international leak in border security.
All corks aside, British Columbia’s wine region has been invaded twice by common wall lizards (that we know of). In 1983, a handful of these invasive lizards were intentionally released in two private gardens in Summerland. Fortunately for the ecology of the Okanagan valley, the Summerland introductions failed. The second invasion, in 2015, consisted of a lone wall lizard found at a vineyard in Osoyoos, transported as a stow-away in a shipment of grapes from Vancouver Island. Fortunately for fans of BC wines, the lizard was removed before grapes were crushed, and died in captivity.
You can see the record in iNaturalist, though the exact location in Osoyoos area has never been pined down.
While writing this short post I tripped across a website for the LacertA Winery in Romania. Their label showcases the eastern green lizard (Lacerta viridis), which grows to 45 cm total length. Imagine if eastern green lizards had been released here instead of the much smaller common wall lizard!
Photos courtesy of Walter Friedl, managing partner, LacertA Winery.
According to Speybroeck et al. (2016), Romania has us beaten for lizard diversity with the snake-eyed skink (Ablepharus kitaibelii), eastern slow worm (Anguis colchica), slow worm (A. fragilis), the steppe runner (Eremias arguta), sand lizard (Lacerta agilis), Balkan green lizard (L. trilineata), meadow lizard (Darevskia praticola), viviparous lizard (Zootoca vivipara), common wall lizard (Podarcis muralis) and Balkan wall lizard (P. tauricus). And now I can’t look at, or open, a bottle of wine without thinking of lizards.
References:
Burke RL, Deichsel G. 2008. Lacertid Lizards introduced into North America: History and Future. 347-353. In: Urban Herpetology. Mitchell JC, Jung Brown RE, Bartholomew B. (eds). Society for the Study of Amphibians and Reptiles. Salt Lake City, Utah.
Conant B. 1945. More reptiles in cork shipments. Copeia 1945(4):233.
Engelstoft, C., J. Robinson, D. Fraser and G. Hanke. 2020. Recent rapid expansion of European Wall Lizards (Podarcis muralis) in British Columbia, Canada. Northwestern Naturalist 101(1): 50-55.
Kraus F. 2009. Alien Reptiles and Amphibians: A Scientific Compendium and Analysis. Invading Nature: Springer Series in Invasion Ecology 4. Drake JA (ed). Springer, New York.
Lever C. 2003. Naturalized Reptiles and Amphibians of the World. Oxford University Press, Oxford.
Speybroeck, J., W. Beukema, B. Bok, J. Van Der Voort, and I. Velikov. 2016. Field Guide to the Amphibians and Reptiles of Britain and Europe. Helm Field Guide Series, Bloomsbury, London, United Kingdom.
I learn new things just by sitting in my garden and watching the behaviours of common wall lizards (Podarcis muralis). Flies may be fast, but lizards nab them. I put cutworm grubs on a pile of rocks and lizards happily sneak up and grab them too. Lizards eat ants.
People tell me wall lizards eat earthworms, even dried earthworms. I have received photos of wall lizards eating wasps. Wall lizards eat each other. They eat their own eggs.
But one thing that is abundant in my garden that lizards leave alone is the ladybug. We have several species of ladybug. The lizards have specific rock piles they seem to hold as resident territory, especially this pair of adults (above)—these two are always close to a small rock pile in my garden.
Right now, those same rocks are covered with larvae, pupae and adult ladybugs. Ladybugs smell bad because they emit:
2,5-dimethyl-3-methoxypyrazine (DMMP)
2-isopropyl-3-methoxypyrazine (IPMP)
2-sec-butyl-3-methoxypyrazine and
2-isobutyl-3-methoxypyrazine
The smell is described as a mixture of nutty, green bell pepper, potatoes and mould. I assume ladybugs taste like they smell. Even the yearling lizards in my garden are leaving the pupae and larvae of ladybugs alone, so they must learn early to leave ladybugs alone.
Even a small number of ladybugs can taint a batch of wine, and since lizards taste their way through the garden, constantly flicking their tongues, it is no wonder they leave ladybugs alone. Lizards likely don’t even have to bite a ladybug to know it is unpalatable.
Ladybugs and wall lizards do however eat the same prey—aphids. Gardeners may be happy to have both of these colourful predators in their gardens.
The chemicals in ladybug emissions are from:
Rovner, S.L. 2007. Why Ladybugs Smell Bad. Chemical & Engineering News. https://cen.acs.org/articles/85/web/2007/03/Ladybugs-Smell-Bad.html
Only one species post
I do prattle on about the invasive common wall lizard (Podarcis muralis) on Vancouver and Denman Islands, and the recent appearance of one Italian wall lizard (P. siculus) in Vancouver, but to put things into perspective, only two lizard species have appeared here, and only one is established. Others from the pet trade, or arriving as contaminants in tropical plant shipments (e.g., bearded Dragon, brown anole, green iguana), represent individuals that escape and never get established. Tropical species will not survive our cool wet winters.
Hawaii, by contrast, has more exotic lizard species than BC has reptiles. The common wall lizard pales by comparison to the Green Iguana.
The books I have for Hawaii are out of date, but they will serve to express the magnitude of the problem posed by accidental import, accidental release from the pet trade, and intentional release in areas where exotic species will survive.
Combined with records on iNaturalist, the lizards introduced to Hawaii include the green iguana, green anole, brown anole, knight anole, Jackson’s chamaeleon, mourning gecko, stump-toed gecko, Indo-Pacific slender gecko, common house gecko, Madagascar giant day gecko, orange-spotted day gecko, gold-dust day gecko, Tokay gecko, delicate garden skink, mottled snake-eyed skink, moth skink, copper-tailed skink and azure-tailed skink. Two species of horned lizard (Phrynosoma cornutum and P. coronatum) were released on Oahu, but failed to establish populations, and the azure-tailed skink probably is extirpated—an invasive ant may have caused its demise.
The green anole, a common pet lizard. This one was in captivity.
If we include species that died out, there are 20 species of lizard introduced to the Hawaiian Islands, with 7 species accidentally transported by Polynesians, and the rest more recently from the pet trade or as stow-aways in packing material. Only the yellow-bellied sea snake and sea turtles are native to the Hawaiian Islands, all other reptiles and amphibians were introduced by humans.
A female common wall lizard in Saanich, Vancouver Island.
I am sitting in my dining right now and looking out at the grey sky, knowing that it is 8 degrees Celsius outside. Tropical lizards have no chance on Vancouver Island, and so I am not alarmed at the annual wave of brown anoles that arrive as eggs in plant shipments from the USA. The lizard species that could survive here need to be able to withstand freezing temperatures. European lacertids, like the green lizard, western green lizard, Ibiza wall lizard, Dalmatian wall lizard and Italian wall lizard that at one time or another were established in the United States, and the viviparous lizard which appeared in Japan, are the only lizards that fit the profile. Perhaps the entire family should be prohibited from the pet trade in North America to limit the risk of introduction.
The western fence lizard also is a species that could survive in BC—it may be in the Okanagan region already, as far north as Oliver—so keep your eyes peeled for these guys. They have also been introduced to the Puget Sound area in Washington and would do fine on southern Vancouver Island. These prickly lizards are common around human habitation. They have bright blue patches on their bellies and do push-ups as a territorial display—if you see one, let me know or tag it in iNaturalist.
After picking up a range of starters at the local nursery, we are finally ready to plant the rest of the beds in the garden. A pumpkin starter arrived from the garden of Steve and Amy Lewis—they started a few too many mega-pumpkins—we were happy to get a seedling. Wasabi is the experimental plant for this year.
Soil was turned over, manure mixed down a bit, and weeds were pulled in preparation for planting. One of the most invasive plants in our front garden is Calendula—ours has a gorgeous orange flower, but this flowering plant had overgrown a third of the front vegetable bed.
Seeds arrived by mail, and we bought a range of peppers, some hotter than others—the scorpion pepper will be interesting.
All our tomatoes went in, as did cabbages, and we have a few hundred New Zealand spinach plants poking up. Ladybird beetles are pupating and there are loads of adults, so let’s hope they keep busy dealing with aphids.
New Zealand spinach (above) has an odd textured leaf and is not to everyone’s liking, but as it’s an edible weed, I am OK with it. Between New Zealand spinach, alexanders, kale, chives, oregano and leeks growing wild in the garden, we will always have some garden fresh food. The lettuce we bought as starters has overgrown its pot!
Lizards are active and emerge by 08:30 as the sun hits the front garden. Two larger lizards seem to have replaced the three yearlings that were on the back fence, and our gravid female still has not laid eggs. Two lizards seem to have been attacked—tails were shortened—but that seems to be it for lizard drama in the last few weeks.
Bewick’s wrens are nesting in our back yard, bushtits are stocking up on spider webs (and the cotton thread we use to tie up plants), crows are building nests, robins have hatched over a week ago, and Anna’s hummingbirds, chipping sparrows and house finches pop by regularly to liven up the garden.
Now to set up the watering schedule and let nature do its thing.
Light rain all day yesterday and the soil is nice and damp, but now warmed by today’s sun. Everything smells fresh and amazing. Lettuce starters are doing really well.
Last year’s kale is in full bloom, ensuring we have kale year round with zero effort. Other flowers do their part to attract bees.
Fruit trees flowering everywhere you turn. Blueberries soon will flower in the front yard.
Strawberries in bloom, rhubarb growing nicely, the fennel is up, and red sorrel pops up where it wants.
Rosemary attracts bees to the garden, while our raspberries and thorn-less blackberries send off many new leaves. Their flowers will soon appear. Oregano is everywhere in the raspberry bed.
Forgotten onions are growing, others have been planted. Potatoes and carrots are planted. Free range chives, kale and chard is as luxuriant as ever.
And our tomatoes—grown from seeds saved from last summer’s successes—are enjoying the sun on our south-facing deck.
And the lizards are increasing in abundance. This one likely hatched out early last summer. I haven’t seen our gravid female in a few days—maybe she is digging a nest.
Bewick’s wrens seem to have staked out territory and may nest here this summer. Chipping sparrows appear daily, as do American robins, dark-eyed juncos, chestnut-backed chickadees, house finches and our resident Anna’s hummingbirds.
Turkey vultures and bald eagles soar in thermals. And Cooper’s hawks keep the smaller birds on edge.
This city garden is alive.
Not much has changed in the garden itself since late March. Some weeding has been done, and I am specifically targeting a few plants that produce seeds early. Purple Deadnettles are everywhere and the battle with them essentially is a Kobayashi Maru scenario. Hairy Bittercress is another I target early since it catapults its seeds early in spring when the seed pods dry. Other than weeding and a bit of cleanup, we are now starting seeds indoors.
Thyme, tomatoes, basil (the seeds were not faulty), cilantro and peas came up nicely.
The arugula and cress are grown as “microgreens” to go directly into salads and sandwiches instead of into the garden. Growing microgreens is a year round thing for us—we have a small light stand with LEDs to start plants from seeds. We also have some flowering plants just for the flowers.
Leftover potatoes and newly bought onions are getting planted this Eostre weekend.
One of my favourite plants in the garden, however, is not something we eat. Mosses—mosses grow like mini forests and while some people power-wash the moss away, I like moss. I wish lawns were entirely made of mosses. Moss is soft on the feet, requires no mowing or maintenance or fertilizer, there is no need for aeration, and it makes plenty of habitat for tardigrades. I may never see a tardigrade in my garden, but I hope they are there and thriving. Since they can survive exposure to outer space, my garden likely is a tardigrade-happy place.
I remember the lifecycle of mosses from my time as a teaching assistant in Intro Biology (course # 71.125) at the University of Manitoba. Moss plants—more specifically, the haploid gameteophyte (gamete producing plant)—we are used to seeing come in a male and female form. The female and male gametophytes produce gametes—egg cells and sperm cells respectively. When water splashes on the male plant, some sperm splashes on top of the female plant, and fertilization of the egg cell occurs. Fertilization of an egg (syngamy) merges each haploid copy of the species genes into one cell (the zygote) which continues to grow with its diploid gene complement.
The sporophyte (or spore producing plant) is the next stage of the plant which grows as a stalk out of the top of the female gametophyte. The stalk elongates and develops a pod (known as a sporangium) at the tip where haploid spores are produced by meiosis. Those haploid spores are released and produce the next generation of haploid male and female gametophytes. This is my memory of the basic moss lifecycle—not bad considering I last taught intro biology labs in the early 1990s.
And I will end this Printemps-post with a gratuitous photo of one of our Common Wall Lizards, one of four (possibly five) which have taken up residence in the front garden. They were basking in the sun at 08:30 this morning.
ROBB BENNETT, CLAUDIA COPLEY, DARREN COPLEY
Abstract
Apostenus ducati sp. nov. is described from montane areas in or adjacent to the Columbia River Basin of southeastern British Columbia in Canada and northern Washington and northwestern Montana in the United States. This is the second Nearctic species of this primarily Palaearctic genus. Unlike most liocranids, A. ducati apparently is restricted to open rocky habitats, such as talus and scree slopes, and on mountain peaks. Throughout most of its range, specimens occur in low numbers and populations are patchily distributed. Also, populations appear to be concentrated in the upper regions of the Flathead River watershed in British Columbia, an area of significant and competing ecological and economic values. Because of these factors, A. ducati is potentially a species of conservation concern.
Keywords
See full article
When a trawl net goes down to the sea floor, you have no idea what will appear when the net is hauled to the surface. Some fish are caught as the net descends, others as the net ascends, but most are caught at the target depth. One thing is for certain, trawl nets sample very specific habitat – open smooth substrates where the net is unlikely to snag.
Since we don’t drag nets over rocky habitat, that habitat is poorly known. You’d think that in the dark depths, substrate wouldn’t matter – but apparently it does. And you’d think we’d already know whether a fish the size of a coffee table inhabited our waters. Not so. Since 2005, we have discovered three new skates in BC, and you guessed it, one was the size of a coffee table. The first Angel Shark for British Columbia was discovered in 2016, and captured on camera, not a net.
The Pacific White Skate (Bathyraja spinosissima) was caught in a trawl net by accident. From footage from submersibles, we know this species cruises about 1 m above rocky habitat. The single specimen caught in 2005 had strayed over net friendly habitat. When this skate was caught, we were unable to assign it to species. Its DNA suggested it was a species from the Atlantic. After a careful measurements, we determined it was a Pacific White Skate, and its discovery was published by Orr et al. (2019).
Bathyraja spinosissima (Pacific White Skate) – this specimen was the first to be included in DNA barcoding which gave us strange results when the tissues were originally sequenced. This large male is the first adult male in museum collections, a first from BC, and extended the known range north from Oregon.
Orr et al. (2019) also listed a second new skate species for BC, the Five-spined Skate (Bathyraja microtrachys). Five males were collected west of Tofino, off Vancouver Island in 2005. These are the only adult males known for this species.
Bathyraja microtrachys (Five-spined Skate) – originally thought to range from Washington south to San Diego, we now known they range into Canadian waters.
In 2009, a third skate species was discovered, the Commander Skate (Bathyraja lindberi), and was published by King et al. (2019). This is a northern skate, and the discovery in British Columbia extends the species’ range south about 600 km.
Bathyraja lindberi (Commander Skate) – appeared in 2009, and now is known from the Sea of Okhotsk, the Bering Sea, Aleutian Islands, south to Queen Charlotte Sound.
Finally, in 2016, a Pacific Angel Shark (Squatina californica) was spotted and photographed by Mark Cantwell while diving off Clover Point, Victoria. The photograph was proof enough of the species’ presence (King and Surry 2016). Since this species was known from a single specimen in Alaska, one from Puget Sound in Washington, and exists south to the Gulf of California, its discovery in British Columbia was only a matter of time. More details on the Angel Shark can be found in Pietsch and Orr’s (2019) magnum opus on Fishes of the Salish Sea.
Squatina californica (Pacific Angel Shark) – was is known from one specimen in Puget Sound, Washington and an old record from Alaska, and finally has been confirmed for British Columbia. Photograph by Mark Cantwell, April 30, 2016 off Clover Point, Victoria, Vancouver Island.
For more details on all cartilaginous fishes in British Columbia, buy the latest RBCM handbook by King and McFarlane due out May 2020.
Non-game fishes receive little attention and of these, the deep sea anglerfishes (Families Oneirodidae, Melanocetidae and Ceratiidae), are among the poorest known. Deep sea anglers live at extreme depths and few people study them.
Alive they are quite elegant with black-brown skin, flowing fins and globular bodies. But when hauled to the ocean surface and preserved in museum collections they tend to resemble shrivelled hockey pucks – with teeth. Most of what we know about these fishes is based on the females. Males are minute but have enlarged olfactory chambers. Males have one goal – to find and latch onto a female. Once attached, males draw nutrition from the female’s bloodstream. Sometimes more than one male attaches to a female, and they are in place ready to mate whenever a female is ready to release eggs. Some have said this relationship is parasitic, and while males do derive nourishment from the female, they fertilize a female’s eggs. In contrast, a parasitic relationships is one-sided. Perhaps the only time the relationship is parasitic is when a male attaches to a female of a different species – he draws nourishment, but provides no genetic contribution.
Because these fishes rarely reach the surface in good shape (trawl nets are not forgiving to soft-skinned fishes), many deep sea anglers are difficult to identify. Identification often is based on the structure and shape of tip of the lure – a structure called the esca – which sits at the tip of the first ray of the dorsal fin. If that delicate structure is lost, identification can be difficult without DNA barcoding. With DNA barcoding, we can identify species based on unique genetic sequences taken from tissue samples. One of the fishes photographed in this article, Oneirodes thompsoni, had been sampled for DNA barcoding to get a representative sequence for this species. The white tag with a code number is attached to the fish so that the fish and its genetic sequences can be matched later.
Until the paper by Weil et al. 2015, only three species, had officially been recorded from BC waters. Specimens of the large Krøyer’s deep sea angler fish (Ceratias holboelli) had been preserved, but no one had detailed where the species had been found. Several other dreamerfish had been misidentified in museum collections. The review of species known to exist here was prompted by the identification of a specimen of Melanocetus johnsonii in the RBCM collection, and the discovery of Oneirodes acanthias and Cryptopsaras couesii in 2006. The other species deemed new to BC, or representing significant range extensions, were discovered during the preparation of the paper by Weil et al. and his review of the RBCM collection.
Below is the list of anglerfish species known to exist in British Columbia (as of March 2020):
Order: Lophiiformes
Oneirodidae:
Oneirodes thompsoni
Oneirodes bulbosus
Oneirodes eschrichtii
Oneirodes acanthias
Chaenophryne melanorhabdus
Chaenophryne longiceps
Melanocetidae:
Melanocetus johnsonii
Ceratiidae:
Ceratias holboelli
Cryptopsaras couesii
Oneirodes thompsoni, RBCM 010-00196-009, 10.9 cm Standard Length (above); and O. bulbosus, RBCM 004-00005-001, 8.8 cm Standard Length (below), are the most commonly caught dreamers along the entire BC coast. Photo by G. Hanke.
Oneirodes eschrichtii, RBCM 998-00323-002, 8.3 cm Standard Length, represents the first of its species in BC and a northward range extension of 1700 km in the eastern Pacific Ocean. Photo by G. Hanke.
Oneirodes acanthias, RBCM 010-00197-004, 10.5 cm Standard Length, represents the first specimen from BC, and a range extension of 600 km north of the species previously known range off Oregon. Photo by G. Hanke.
Chaenophryne melanorhabdus, RBCM 999-00107-002, 8.9 cm Standard Length, is another commonly caught dreamer, but we have no records north of Vancouver Island. Photo by G. Hanke.
Chaenophryne longiceps, RBCM 998-00344-001, 9.8 cm Standard Length, is one of three specimens now known from BC, roughly 600 km north of the species previously known range off Oregon. Photo by G. Hanke.
Melanocetus johnsonii, RBCM 01400308-001, 9.8 cm Standard Length, is the only specimen known from BC waters and is the first record north of Oregon on this side of the Pacific. Photo by G. Hanke.
Ceratias holboelli, RBCM 999-00081-001, 42.0 cm Standard Length, is our largest deep sea anglerfish, distinctive with its thick elongate fin rays and cover of large thorny denticles. Photo by G. Hanke.
Cryptopsarus couesii, RBCM 014-00309-001, 14.2 cm Standard Length, was caught as bycatch from the commercial fishery and since it was so strange, was kept for identification. It represents the northern most record for the species in the eastern North Pacific Ocean and a first for BC. Photo by G. Hanke.
Spring has sprung, and it is great time to get going on a garden. There are a ton of things you can grow at home to supplement your diet and reduce your dependence on grocery stores. My wife and I have jokingly named our property UF1510 (Urban Farm 1510). Our street address obviously is 1510, and our garden is the only one on this street fully devoted to food production.
Planting seeds for this summer.
When we bought the house over 10 years ago, the front yard had a small patch of St. John’s Wort, a spindly ornamental shrub, a sizable Laurel tree, an isolated Camelia bush and a dodgy coniferous tree. But the majority of the front yard was a flat expanse of lawn. Everything but the Laurel was removed quickly, and over the years, the lawn has been eliminated. We have dedicated a third of the front yard to bare ground for potatoes, beans, carrots and a few other crops. The remaining two-thirds is a “food forest.” We planted blueberries, sour cherries, apple trees, hazelnut bushes, asparagus and a range of flowering plants to attract pollinators. Don’t forget the bees—if you want tomatoes, berries, cucumbers, squash—you need pollinators.
Alexanders (left) and Salad Burnet (right) are available in winter as a supplement to salads.
The back yard also was a monoculture of grass when we moved in. It now is changed to a series of raised beds and we grow many varieties of tomatoes, kale, chard, onions, leeks, chives, cucumbers, squash, blackberries (a thornless variety) and raspberries—to name just a few of our home-grown food items. Many of our plants self-seed and effectively are edible weeds (cilantro, salad burnet, alexanders, arugula, kale, leeks and chives are weeds in our garden). I also eat dandelion leaves.
The back yard with its raised beds just waking up from the winter. Oregano, chives, raspberry canes and leeks are going strong—we will have to wait for the raspberries of course.
This change in gardening practice took us from a flat lawn which needed to be mowed regularly (wasted energy, in my opinion) to a garden we can walk around and pick fresh produce for our daily meals. Even in winter (because we live on southern Vancouver Island), there is food available in the garden. We also leave the deadfall from the previous summer in place all winter. Why leave the deadfall in place?
1) I am lazy.
2) it is habitat, shelter and a source of food for overwintering birds.
Our garden is chemical-free so there also are insects—but thanks to our local birds (and the invading wall lizards), we get free pest control, entertainment and a dash of colour as they flit about.
Rhubarb, kale, chard, rosemary, and winter cress seem to look after themselves.
This year I am going to post a series of blog articles to show the progress in the garden—this early in the spring it looks messy—weeds have grown, and last summer’s deadfall has yet to be cleared away. But the garden is full of life—birds are everywhere. Lizards skitter around on the warmer days (they invaded in 2019 from a small population to the north of us). We welcome spiders, but we do draw the line at rabbits and deer – they are not permitted.
Strawberries, a potted peach tree and some early flowers.
This month, we will clear out the dead stems and weeds. The dead stems go into the compost—they form the dry brown material that alternates with wet kitchen scraps. We don’t eat meat, so everything from the kitchen goes into the compost. And in the kitchen we have light stands to start seeds. Once the risk of frost is gone, the seedlings will be planted.
This summer’s tomatoes will be started indoors.
I hope this series inspires people to change their gardening practices and replace lawns with food plants. Even a deck or apartment balcony can provide a small mountain of food. You can even grow your own sprouts in your kitchen to add some home-grown greenery to a sandwich. Our garden is a green security blanket, a source of exercise, entertainment and also nourishes us with food and a sense of pride.
Tonight, we are having homemade soup which will have diced leaves of home grown kale, chard and alexanders. To kick the series off, here is the recipe for the soup.
2 chopped carrots
1 chopped medium sized yellow onion
2 chopped celery stalks
3 cups of vegetable broth plus 3 ½ cups of water
1 pack of simulated shredded chicken
about 1 teaspoon of poultry seasoning (I never measure exactly, this is cooking not chemistry)
about a 1 cup of pasta or 1/3 of a package of spaghetti or linguini
1 cup or so of frozen peas
We sauté the carrots, celery and onions in a large pot on medium heat for 5 or so minutes. Then we add the broth, the 3 ½ cups of water, fake chicken, and seasoning and bring to a boil, then simmer for 10 minutes – maybe more – until the veggies are cooked. Then toss in the pasta and peas and simmer until the pasta is to your liking, and then we add a handful of chopped kale, alexanders, and chard at the end. Season with salt, pepper to taste. I also add a few drops of a decent hot sauce to my bowl.
Thank you garden.
Not a Killer Whale? post
When is a whale not a whale? When taxonomy conflicts with everyday language.
According to the author of a recent article on the internet, “Although they’re called killer whales, orcas are not actually whales; they belong to the oceanic dolphin family, of which they are the largest members.”
That statement is both correct in that orcas are in the family Delphinidae, and also incorrect at a higher level—and such statements are ubiquitous on the internet. Using this logic, a goldfish is not a fish because it is a member of the carp family. You are not a placental mammal because you are in the family Hominidae. And roses are not plants because they are in the Rosaceae.
Carp are fish. Minnows are fish. Goldfish are fish. Placing species in family groups in manmade taxonomic schemes does not eliminate the larger more inclusive groups defining types of organisms. A goldfish is a cyprinid, cyprinids are a type of fish, and therefore, goldfish are fish.
Orcas presently are in the family Delphinidae. The family Delphinidae is one of many cetacean (whale) families. So by the transitive relations: orcas are whales.
Whales ultimately evolved from a group of terrestrial mammals, derived from synapsid reptiles, which evolved from amphibians, which evolved from lobe-finned fishes. So mammals, including whales, also are a subset of fishes, if we take this to a crazy extreme. I take great delight in being a fish and a Pisces—not that the present alignment of stars is an indicator of anything.
In 2017, the BC Archives acquired an Emily Carr watercolour of a woodland scene (PDP10276, Fig. 1). The work is an example of Carr’s early interest in the landscape of British Columbia. Recently, the work made its way back to the Paper Conservation Lab to receive the treatment that was recommended at the time of acquisition.
When this work arrived at the Royal BC Museum, a few condition issues were apparent. There was some evidence of fading, a small stain in the upper left corner and some microscopic cracking of the paint layer (Fig. 2), but these issues are irreversible and— now that the painting is entering a stable, museum storage environment—not particularly worrying. There was, however, one big (and unsightly) problem: the painting had been adhered to a low-quality, acidic cardboard support at some point in its history, and that support layer had been partially torn away (Fig. 3).
Why is this a big problem?
Mechanically:
Chemically:
Treatment
The solution was clear: this partial backing had to be fully removed.
Removal of a backing usually happens in stages. The backing board attached to this painting was rigid and thick. Trying to remove something thick from something thinner all at once will usually result in tears, delamination and/or creasing in the thinner, more flexible object—in this case, the painting. To avoid this, the thick backing is removed in thin layers. The backing is reduced, layer by layer, until we reach the final layer, which is attached to the painting. This final layer is the one carrying the adhesive, which makes it the trickiest layer to remove and the layer that needs to be removed most delicately.
The initial layers were removed using a thin, slightly dull blade. Upon reaching the final layers, it was apparent that there were many areas, particularly towards the outer edges, that were not directly adhered to the back of the painting. These areas lifted up easily without the blade.
The remaining, adhered portion was tested with a small amount of distilled water under the microscope and fortunately, the adhesive was discovered to be water-soluble. To be sure that the application of water on the back would not solubilize the media on the front, the media on the front was tested under the microscope with small drops of water (Fig 4). Each different colour was tested in discrete areas. Fortunately, only one colour was partially affected by the water. Given the thickness of the paper and the sensitivity level of the paint, it seemed unlikely that the water used for the backing removal would cause damage, and therefore a small, controlled application of water, administered under the microscope, was used to remove the final layer of the backing.
Following the backing removal, a small tear along the left side of the work became unsupported and therefore, unstable. To prevent this tear from growing bigger, it was repaired by pasting a thin piece of Japanese tissue over the tear on the back (Fig 5). The Japanese tissue is made of long natural fibres, which makes it very strong and supportive, despite how thin and translucent it appears after application.
Now that the work is physically and chemically stable, it is ready to be mounted so that it can be made accessible for research and exhibit (Figs. 6 and 7). Once the work is matted and framed, the change from “before” to “after” won’t be detectable to most viewers, but this work is essential for the long-term stability of the painting. It is an invisible gift that this generation bequeaths to the next.
New Eels in BC post
Prior to 2010, the eels of British Columbia had been poorly studied and only 6 species of eels and eel-like fishes were known from our coastal waters. Two species of cutthroat eel (Histiobranchus bathybius and Synaphobranchus affinis) were expected to appear here because they range from the west coast of the contiguous United States to Alaska, but as of February 2020, neither species has been discovered in British Columbia.
Kamikawa and Stevenson (2010) led the charge with the publication of range records for Aldrovandia oleosa, including those from British Columbia. Their new records from 819-2014 m off California and British Columbia represent a range extension of 11,000 km from the nearest historic records in the western central Pacific Ocean.
Hanke and Roias (2012) announced the discovery of two duck-billed eel species in British Columbia. As of January 2020, only one specimen of Venefica ocella has been found in BC waters, off the southern end of Haida Gwaii at 1669 m. Five specimens of V. tentaculata have been found at depths of 1757 to 1869 m, from northwest of Haida Gwaii to Vancouver Island. These specimens extend the range of the genus at least 900 km north in the eastern North Pacific Ocean and suggest these eels also range north into Alaska.
Hanke et al. (2014) added another 4 new species to the fish fauna of British Columbia, and updated the known ranges of another 4 species. The Snub-nosed Spiny Eel, Notacanthus chemnitzii, had been caught repeatedly in British Columbia, and had been noted in several checklists (Gillespie 1993, Love et al. 2005), but details of the species’ range, and the depths it inhabits (1480 to 2400 m) along our entire coastline went unpublished until 2014. The first specimen of Synaphobranchus brevidorsalis for British Columbia was caught in 2006 at 1751 m off the north end of Vancouver Island and is significant as the first record for the North Pacific. Nemichthys larseni, the Pale Snipe Eel, was caught roughly 18.5 km inside the Canadian Exclusive Economic Zone at a maximum depth of 300 m, and to date is the only specimen from British Columbia. Hanke et al. (2014) also extended the range of Cyema atrum (the Bobtail Eel) north from Oregon to British Columbia with the 2006 collection of a single specimen from 1890 m off the north end of Vancouver Island.
There have been no other eel species discovered in British Columbia waters since 2014, and below is the list of 13 spiny eel, gulper eel and true eel species known to exist off here (as of February 2020):
| Order: Albuliformes | |
| Halosauridae: | Aldrovandia oleosa |
| Notacanthidae: | Notacanthus chemnitzii Polyacanthocephalus challengeri |
| Order: Anguilliformes | |
| Colocongridae: | Thalassenchelys coheni |
| Congridae: | Xenomystax atrarius |
| Serrivomeridae: | Serrivomer jesperseni |
| Synaphobranchiidae: | Synaphobranchus brevidorsalis |
| Nemichthyidae:
|
Avocettina infans Nemichthys larseni Nemichthys scolopaceus |
| Nettastomatidae: | Venefica ocella |
| Venefica tentaculata | |
| Order: Saccopharyngiformes | |
| Cyematidae: | Cyema atrum |

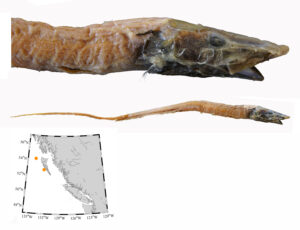
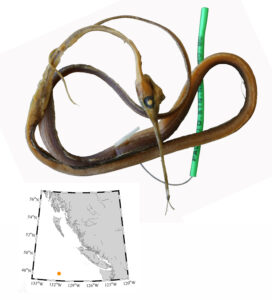
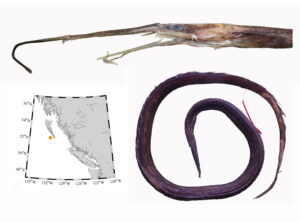
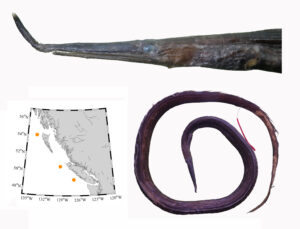
Warm Water Strays post
Most new fishes found in the last two decades were caught during deep water surveys and are adapted to cold water – they probably are not new, just newly discovered. However, marine life is known to stray north during el Niño years, and with the warm water “blob” events in the last decade, unusual northward range records were expected. But northward strays are not a new phenomenon, with historic records of smooth hammerhead sharks (Sphyrna zygaena) off Vancouver Island (Carl 1954, McFarlane et al. 2010), and a tiger shark (Galeocerdo cuvier) and finescale triggerfishes (Balistes polylepis) as far north as Alaska (Mecklenburg et al. 2002).
Only a few of the new range records in BC likely are attributable to warmer surface waters, including the appearance of the North Pacific argentine (Argentina sialis), which historically was known north to the mouth of the Columbia River in Oregon (Love and others 2005). In 2011, a single North Pacific argentine was caught west of Clayquot Sound from 118 m, and represents the first record of the species in British Columbia.
Three years later, during the height of the 2013-2015 warm blob event, a finescale triggerfish (Balistes polylepis), was caught alive and well off the west side of Vancouver Island, and a fresh louvar (Luvarus imperialis), was found on the beach near Massett. The louvar and finescale triggerfish represent significant strays north of Baja. With its body evolved for open water cruising, it is easy to see how the louvar made its way this far north, but even triggerfishes which swim at a leisurely pace, have strayed into southern Alaska during el Niño events.
Jump forward 5 years, and a spotted porcupinefish (Diodon hystrix) was found dead at low tide, Jordan River, southern Vancouver Island. The specimen was fairly fresh and had not been scavenged. A second porcupinefish was seen on that same stretch of beach on September 26th (Jerrett Taylor pers. comm.). The second fish was not recovered. Following the coastline, the fishes found at Jordan River are about 2100 km north of their previous range limit off San Diego. It seems unlikely they swam along the coast against the California Current, where upwelling keeps the water cold, but instead took an offshore route in water 2-3°C warmer than normal. Open water excursions are possible given that spotted porcupinefish have crossed open ocean to reach Hawaii, Pitcairn, Easter, and the Galapagos islands. Spotted porcupinefishes have an long larval phase in open water and according to Leis (1978), this is ample time for dispersal.
Were these the first porcupine fishes in BC? Yes. But a relative of the porcupinefish, a burrfish (Chilomycterus affinis; RBCM 610), was found in BC in July 1939, along Dallas Road in Victoria. The specimen looks like an inflated curio from a tourist trap and Carl and Wilby (1945) were suspicious and did not put the burrfish on the list of fishes known to inhabit our waters.


New Cusk Eels for BC post
Cusk-eels and brotulas of British Columbia have been poorly studied, and until recently, only two species were known from our waters – Spectrunculus grandis and Brosmophycis marginata. However, deep-sea survey samples from 2002-2006, and the commercial fishery provided six new species, with a seventh revealed during re-examination of museum specimens.
One specimen of Cherublemma emmelas was identified from commercial fishery bycatch in July 2006. It was in a trawl haul from 1097 m in Kyuquot Canyon, west of Vancouver Island, and extends the species’ range roughly 2890 km into British Columbia.
Shrimp survey samples, and the commercial fishery in the southern Strait of Georgia, British Columbia revealed an additional cusk-eel species (Chilara taylori) at depths of 78 to 109 m. These two fishes have since been joined by others added to the collection, and represent a modest extension of the species’ range north of Willapa Bay, Washington.
A single specimen of Acanthonus armatus was taken during deep-sea research collections from near Triangle Island at 1778 m and is the first record for the eastern North Pacific Ocean as well as Canada. The announcement of this fish’s presence here netted some unexpected attention because of its common name, the Bony-eared Assfish. The rest of the new cusk eels failed to draw the same level of attention.
Four specimens of Bassozetus zenkevitchi were collected from depths of 1909 to 2125 m west of Vancouver and Graham islands, show the species ranges along our entire coast. It is likely that others were caught years ago, but were mistakenly identified as Arrowtail, Melanonus zugmayeri, and discarded.
A single specimen of Cataetyx rubrirostris taken from 2000 m, roughly 18 km east of the Tuzo Wilson Seamounts in Queen Charlotte Sound, represents the first record in British Columbia and a 750 km northward extension from the previous known occurrence west of Nehalem Bank, Oregon. And as of January 2020, only one specimen of Porogadus promelas is known from BC waters, taken roughly 22 km east of the Tuzo Wilson Seamounts in Queen Charlotte Sound. This fish extends the species’ range into the eastern North Pacific, and also represents the northern-most record of the genus in the Pacific Ocean. The genus Porogadus presently is being revised, and since the RBCM specimen is in such good shape, it is being studied by experts in Copenhagen
During the preparation of the manuscript detailing these new records, I re-examined cusk eels in the Royal BC Museum collection. In the process, a single specimen of Spectrunculus crassus was identified from among the few S. grandis preserved at the Museum. Spectrunculus crassus had been split from S. grandis in 2008, and to date, is the most recent addition to our cusk-eel diversity.
Because of increased sampling effort since 1999 and re-examination of museum specimens, we now know that 9 species of cusk-eel live in our region.
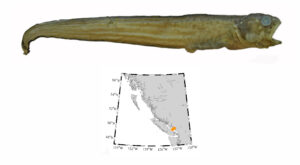

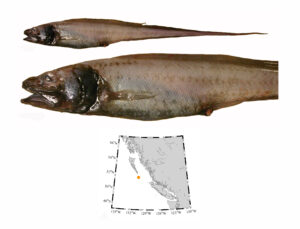
How Handy is That? post
During the yuletide season of 2014, Rhapsody was found dead. Her unborn daughter had died days earlier. Rhapsody was unable to expel her calf and died from the decay and infection. Her calf was examined at the pathology labs in Abbotsford, then returned to Vancouver Island so that we could prepare her skeleton. She sat in a freezer while we debated the best way to prepare so delicate a skeleton.
In the spring of 2019, Mike deRoos of Cetacea Inc. decided we should immerse the calf’s remains in a tank of warm water which was oxygenated with an airstone, and let bacteria do the work for us. Once the tank was ready, I drove to Saltspring Island with a fragrant tub in the back of my car.
Months later, the bones were ready for pickup. My car is nicknamed Tydirium – after the shuttle from the Return of the Jedi – it still smells new inside. Fortunately, the car still smells ok after shuttling a foetal orca back and forth from Saltspring Island.
Now I have the task of sorting out all the disarticulated bones and putting them back in order. Rhapsody and her calf will be displayed alongside each other in our Orcas exhibit opening May 2020.
Like humans, bones of a newborn are still growing – and skull bones are not solidly sutured together. The skull bones from Rhapsody’s calf came apart. Vertebrae are not fully formed either – the neural arches, lateral processes, centra and cookies are all separate. What a puzzle! Ribs, sternal ribs – all of these need to be sorted out. And then there are the finger bones in the flippers – I was not looking forward to sorting these out.
But here is where Mike deRoos saved the day – he arranged to have the calf’s flipper x-rayed, and the image printed at full size is better than an IKEA or LEGO instruction booklet to guide reconstruction of both left and right flippers.
The three carpals and a few of the distal phalanges are not formed – and the metacarpals and phalanges are widely separated by cartilage. But the size and shape of each bone is so distinctive, that I was able to place each bone where it should go in minutes. Two phalanges from the fingertips of the right flipper were lost during preparation – but overall the flippers look great.
Ribs are next.
The BC Archives is currently finishing up a project to arrange, describe and digitize a collection of photographic holdings from Ernest Crocker, a notable nineteenth century photographer. Part of this effort has involved some conservation work to repair some of the elements of these collections that have not made it through the ravages of time in one piece. In this particular example, the image identified as J-02623, there were a great many pieces. The following is a description of how it was repaired.
Background and terminology
Glass plates were used as a support for photographic negatives early on in the history of photography and continued to be used well into the first half of the twentieth century. The negative is composed of two layers: the glass support and the photographic emulsion that holds the image (Fig 2). In the case of this example, the emulsion layer was made from gelatin.
Condition issues
When J-02623 arrived in the conservation lab, it was in many pieces and was resting on a second, intact plate. The fractures radiated from a central point near the upper right corner, suggesting that the negative had been struck by something blunt at some point in its history. In some of the areas around the cracks, the glass had been reduced to dust, which meant that there would likely be small losses once the dust was cleared. Its fragmented and fragile condition meant that it could not be removed from the supporting negative underneath it, which meant that the archivist could not see or identify either image and absolutely couldn’t digitize them.
These condition issues posed a few interesting problems in terms of developing a conservation treatment:
1. Broken plate cannot be stabilized as it is
Cracks in glass plate negatives are not uncommon. In many instances, simple solutions such as custom storage enclosures or pressure mounts are sufficient for long-term stability. Pressure mounts—two new pieces of glass put on either side of the broken original and taped together—were used on several other broken plates within this collection. The benefit of this method is that the new glass provides the necessary support for the plate to be handled again and the treatment is completely reversible, allowing for reconsideration of treatment alternatives in the future if desired. In the case of J-02623, however, this would not be feasible—the negative was too fragmented and would not be adequately supported by a pressure mount alone.
2. Broken plate cannot be moved as one piece
Before methods for consolidation and long-term storage could be considered, the issue of simply removing the broken plate from the intact plate beneath it had to be addressed. As previously mentioned, areas around the cracks had disintegrated into dust. This meant that sliding the broken negative off of the supporting plate and onto a new piece of support glass would be impossible. Some of this sharp, glass dust was likely to be under the broken shards of the negative. If sliding was attempted, the glass dust would act as sandpaper, abrading the delicate emulsion on the underside of the broken negative, leading to partial losses of the image. It would also likely scratch the glass side of the other negative as well.
3. Reassembly after any disassembly would be very tricky
If the broken pieces couldn’t be slid onto a new support or work surface, they would have to be lifted. But this solution posed its own problems: how do you replace so many tiny pieces back in proper alignment once they’ve been disassembled?
4. How to stabilize for the long-term
Once a plan to remove and reassemble the broken plate is achieved, how do you stabilize it for gentle handling and digitization? If a pressure-mount system is not enough, the only other option is to adhere all of the shards back together; however, this is easier said than done. How do you apply adhesive to glass shards without it oozing out around the edges? What adhesive will provide a strong enough bond while also having good aging properties as well as being reversible/adjustable?
Treatment

Figure 6. Paper conservator Lauren Buttle applying adhesive to glass side of J-02623 using Silpat® method. A respirator mask was required for this treatment because of the use of solvents.
A carefully considered treatment plan was required to answer so many questions.
To achieve the first task of moving the broken plate to a new working surface, the damage was first mapped (traced) onto a clear sheet of plastic with permanent marker and photocopied onto paper. This map was used as the basis for recording the location of each of the tiny shards that would have to be replaced afterwards. Tiny shards that were big enough to carry part of the emulsion were removed one-by-one, placed in a numbered bag and their shape and location was noted on the map (Figs. 4 and 5).
Before the shards were reassembled, the edges of each one were swabbed with acetone to clean away any dirt or oil that might impede adhesion. The shards were reassembled, emulsion-side down, on an inclined surface against a Silpat® mat (a reusable silicone-covered textile designed for baking). This mat provides a non-stick surface that is not completely flat. The texture in the textile provides a series of small bumps that raise the glass slightly off of the rest of the surface. The adhesive is delivered to the cracks on the glass side and capillary action draws the adhesive through the crack. Because there is no consistent contact with a flat surface on the emulsion side, the capillary action stops at the emulsion side, keeping the edges of the cracks on that side nice and neat. All of the pieces were assembled dry first. After all pieces were aligned, adhesive was administered.
After the adhesive in the cracks was dry, the glass-side surface was cleaned gently with solvents to remove any adhesive residue from the edge of the cracks.
The adhesive used for this repair was Acryloid B-72, a non-proprietary acrylic adhesive with known aging properties and good long-term stability. Acryloid B-72 can be dissolved in many different organic solvents. In this case, toluene was used, because it has a relatively slower drying time, which allows more time for the solvated adhesive to flow into the cracks before drying. Various concentrations were tested until full penetration of a crack in a test piece of glass was achieved (Fig. 7).

Figure 7. Test glass with different concentrations of Acryloid B-72 in toluene applied to a crack. When light is shown over the crack, the areas where the adhesive has been drawn in begin to disappear.
After all the pieces had been assembled and adhered in place, the plate could be lifted and handled gently. But due to the larger size of the plate and the amount of cracking, additional support would be needed for long-term care and access. Also, there were small losses in areas in the center of the cracking, which left jagged edges exposed. For these two reasons, it was decided that a pressuremount would be the best way to protect the plate and the patron.
Two new sheets of glass, cut to the same dimensions as the negative, were cleaned and placed on either side of the repaired plate. All four edges were taped to seal the package and allow for easy handling. The plate will be transferred to a member of our Preservation team for digitization with the rest of the collection.
For more information on this collection, see the Ernest Crocker Fonds (PR-1348) on the BC Archives website.
Acknowledgements
The techniques for repair discussed in this blog were based on the methods conveyed by Katherine Whitman, conservator of photographs for the Art Gallery of Ontario (AGO) at a workshop hosted by the AGO in October 2018. My attendance at that workshop was generously sponsored by the Koerner Conservation Initiatives Fund through the AGO and the Royal BC Museum. I am also grateful for the ongoing communications with Katherine Whitman in devising a treatment for this plate.
Thank you to Joel Blaicher, exhibits fabricator for the Royal BC Museum, for building the support that was used in this treatment. Thanks also to Rob Anderson, lighting and AV specialist for the Royal BC Museum, for his assistance in creating a time-lapse video of this treatment.
Tom Bown, Volunteer Archaeology Research Associate.
The first artifacts to arrive at the Royal British Columbia Museum from the Esquimalt Harbour Remediations dredging project (Esquimalt Harbour Remediation 2019) were a pair of pop or mineral water bottles from Charles Mumby, Portsmouth and Gosport.
This establishes a direct link between the Royal Navy Base in Portsmouth and Esquimalt.
As many of the Royal Navy ships would have been resupplied at Esquimalt, bottles and other artifacts from British Columbia companies will likely be found in Portsmouth.

Fig. 1 C. Mumby glass bottle from Esquimalt Harbour, DcRu-1278:125
(photo by author)
The first of these two bottles, is a short squat, amber coloured, cylindrical, bottle with a hand finished blob top. It is 15.5 cm. in height and 7.7 cm in diameter. Remarkably the cork is still in the neck and the bottle might still have the original contents. The front is embossed C. MUMBY & Co with a double circle embossed TRADE MARK P & G and a fouled anchor in the center. A bottle such as this would likely date between 1884 and 1910. Hannon and Hannon (1976) state the term trade mark on Mumby’s bottles post dates 1884.

Fig 2a C. Mumby “torpedo” bottle from Esquimalt Harbour DcRu-1278:36 (front). Photo by author

Fig 2b C. Mumby bottle from Esquimalt Harbour (reverse side). Photo by author
The second, is a “torpedo” shaped bottle also known as a Hamilt on style named after the inventor. In aqua coloured glass, it has a hand finished blob top. It is 19.0 cm in length and 6.1 cm at its widest diameter. On the front it has a double circle with TRADE MARK at the top and P&G at the bottom with the fouled anchor in the center the same as the one in figure 1. Above the circle only the last two letters are legible as ER the phrase was likely SODA WATER MAKERS and below the circle TO HER MAGESTY THE QUEEN. On one side it’s embossed C MUMBY & Co PORTSMOUTH AND GOSPORT on the opposite side. This bottle likely dates between 1884 and passing of Queen Victoria in 1901.
The torpedo style bottle was initially designed to withhold the pressure of the carbonation as well as keeping it from standing up right allowing the cork to dry and the carbonation to escape. They were used extensively in the mid 19th century and a few companies kept the design until the start of the 20th century. In British Columbia, there are no known bottlers that ordered torpedo style bottles embossed with company names.

Fig. 3 Colonel Charles Mumby. Photo with permission of David Moore, Historic Gosport
Starting on the mid-19th century, both the Royal Navy and British Army were recognizing alcohol as a serious problem. Lloyd and Coulter (1963:96) state: “Heavy drinking especially on shore, diminished towards the end of the century when tea and coffee were fast supplanting grog…” Increased levels of technology required higher levels of training that had little tolerance for drunkenness. In addition, the temperance moment was having an influence. As a result, many of the Royal Navy Clubs and regimental canteens were supplying their own soft drink bottles with club names and regimental crests (Bown 2015). A second advantage of these products was the carbonation which acted as a preservative keeping the contents for long sea voyages.
It would appear Charles Mumby saw an opportunity and was close at hand to supply the Royal Navy with a good supply of non-alcoholic beverages. Even his choice of logo with the fouled anchor was likely designed to appeal to the Navy.
Like many prominent businessmen in the Victoria era, Mumby was an officer in the local militia. This may have given him the necessary status and connections to supply the Royal Navy. The following was provided by David Moore at Historic Gosport (2019):
“Charles Mumby set up business in Gosport in 1849 as a chemist and manufacturer of mineral waters. His shop was at 47/48 High Street. His supply of water was a large bore hole in the yard at the back of the shop, which had rear access from North Street. At 345 feet he hit natural water in the chalk subsoil. He installed machinery to increase the production of natural ice. He produced famous soda water, ginger beer and lemonade, selling across the south of England. He supplied the Army and the Navy, receiving a Royal Warrant from Queen Victoria. The manufacture of mineral waters continued at his original premises in the High Street, and an office was opened up at Portsmouth, first at 71 St George’s Square, then, from the late 1870s, at 34 The Hard. Charles Mumby was a Poor Law Guardian, a magistrate, a County Councillor for Hampshire, and sat on innumerable public and social committees. Charles retired in 1885 leaving the business to his son Everitt.” It was floated as a company in 1898.”
WikiTree (2019) also states Mumby and Company was awarded a Royal Warrant to supply King Edward VII and the company continued to supply the Royal Navy until about 1970.

Fig. 4 A Photo of Mumby’s Shop, Photo with permission of David Moore, Historic Gosport
Located at 47/48 High Street sometime after the reign of Queen Victoria as the banner states TABLE WATER MAKERS TO HM THE KING. His business in Gosport was conveniently located within a kilometer of the Royal Navy base at Portsmouth across the harbour.
References
Bown, T. and C. Addams, 2015. Glass and Pottery of the Royal Navy and British Military: Historic and Archaeological Finds form the 18th, 19th and 20th Century. First Choice Books Victoria B.C.
Esquimalt Harbour Remediation 2019.
https://www.canada.ca/en/department-national-defence/news/2016/09/esquimalt-harbour-recapitalization-remediation-projects.html Accessed July, 2019.
Hannon T., and Hannon A., 1976. Bottles Found in St Thomas, Virgin Island Waters. Journal of the Virgin Islands Archaeology Society, Volume. 3, 1976, pp. 29-46.
Historic Gosport, 2019. David Moore Webmaster.
https://historicgosport.uk/bottles/ Accessed July, 2019.
Lloyd C., and Coulter J. L. S., 1963. Medicine and the Navy 1200-1900, Vol IV 1815-1900. E. & S. Livingstone Ltd. Edinburgh and London.
WikiTree 2019.
https://www.wikitree.com/wiki/Category:Charles_Mumby_and_Company. Accessed July, 2019.
Historic Objects from the Esquimalt Remediation Dredging Project
by Tom Bown, Volunteer Associate in Archaeology
Nattering post
We rely heavily on technology – integration – connectivity. This was the downfall of the newer generation of Battlestars when the Cylons attacked. Today the government network was down. But it was still possible to connect my phone and a memory stick and write something. And today I am writing about an integrated, connected global network for biological information which at the moment, is inaccessible to me.
For a few years now I have been collecting lizard range records in a spreadsheet to support a research paper on rapid range expansion in the Common Wall Lizard. That paper now is in-press and will be printed this autumn. Now that we don’t need to be so secretive with our data, we have changed strategies and are encouraging people to report lizard sightings directly to iNaturalist.
iNaturalist is a global initiative where anyone can sign up, and then report the species they find. A global network of experts help make sure identifications are accurate, and the combined efforts of thousands of observers creates incredible maps for each species. Obviously the species reports are biased – the maps detail where humans go, and microscopic life and invertebrates are underrepresented because they are really hard to ID from a simple photo. But fungi, larger insects, plants, vertebrates – all are mapped in amazing detail.
These location records now are available to people around the world to track species ranges and how species ranges may change in the future – climate change, habitat loss – you name it – the data is out there to study.
To enter a record, first you need to get a photograph –you can make a report without a photo – but it is impossible for others to verify what species you found. Photos need to be as good as you can get – a fuzzy blur is not much help. You also can report a sound recording (bird calls for example, or maybe sound files from bat detectors).
This weekend my wife and I were walking around Butchart Gardens on Vancouver Island, and the organism I found – no surprise – is a Common Wall Lizard. In the case I detail below, I have iNaturalist on my phone, and this series of images depict the process of discovery to creation of iNaturalist record. The process takes seconds.
You take the photos – and this can be tricky – some animals are skittish. I get a basic shot and then slowly close in to get better photos until the subject runs away (botanists and mycologists don’t have this problem). Even a fairly bad photo like the one below is good enough in some obvious cases- but better photos makes identification easier.
Link the photo(s) to the record.
Then you specify what species you think you have found – or you can leave it to genus, family, or blank.
Then you specify the location and date – you can do this manually, or the software draws the location and date from metadata in your photographs.
Then click on the submit record button – and the record now is live for others to view.
If your identification is correct, the global community will also ID the organism as you did, and the record goes to a status known as ‘research grade’.
If you are wrong, people suggest other identifications. You can choose to agree or let the global community of experts come to consensus. Several of my spider and insect identifications have been corrected by experts – and that is the power of this network. We help each other refine the accuracy of these data.
When I take off with my phone to make iNaturalist records, I say I am Nattering. May has well have a fun name for this activity. It is kind of like trophy hunting – but you create a digital record for the world to see rather than a stuffed animal, or pressed plant, or pinned insect. It’s also highly educational – I have no idea of the identity of many of the insects I photograph, but the software and global community really helps teach what is living right in my own neighbourhood.
It costs nothing to load and use the software and for my wife and I, we use it as a fun activity we can share while enjoying hikes on this Pacific island, or while our car charges during road trips.
Why not join the team and photograph what ever you want – starting with Common Wall Lizards and plot their locations in this software. It is fun and you’d be contributing to science.
Haq & History post
Punjabi Canadian Legacy Project Community Event
Museums across the world are going through paradigm shifts – a shift that calls for a transition of power and authority, and a transition from exclusions to more holistic models of inclusion. The Royal BC Museum has been working towards such shift through community history projects based on community engagement.
In this type of community-based heritage work, the goal is to work closely with previously marginalized groups for exploring, preserving and sharing self-identified communities’ heritage defined in their terms. Our hope is to facilitate the sharing of the self-identified communities’ diverse stories and heritage in our galleries and beyond from community perspectives and in community voices.
The Punjabi Canadian Legacy Project (PCLP), a partnership project between the Royal BC Museum and the South Asian Studies Institute at the University of the Fraser Valley, is one of the museum’s attempts toward this goal. Six years ago in the summer of 2013, the initial discussion that eventually led to the creation of the PCLP took place in the Fraser Valley. Almost four years ago in 2015, the PCLP Advisory Committee met for the first time, where Mo Dhaliwal initiated a call for a revolution to break traditional institutional boundaries. It led to the community gallery intervention event. At this time, Punjabi Canadian communities took over the museum space and inserted their stories and presence.
Years later, community members gathered at the Royal BC Museum again on May 4, 2019, to view the cumulative result of the long-term work – the bilingual Pocket Gallery exhibit Haq & History: Punjabi Canadian Legacy Project a Quest for Community Voices, and the kiosk featuring community voices in the Logging area in the Modern History gallery. The kiosk was installed based on community feedback from the intervention event, sharing family, union and industry stories in the sawmill industry, an industry that have woven the Punjabi Canadian stories in BC together.
Leading this community gathering in the museum, Mo Dhaliwal, on behalf of the PCLP Advisory Committee, started with the land acknowledgement, and thanked all ancestors, matriarchs, and all living things for getting us to where we were. Ravi Kahlon, then Parliamentary Secretary for Sport and Multiculturalism, acknowledged all the PCLP advisors and community participants. The most moving part of the event was when people saw their stories and images, interwoven into the community stories under the five themes. The five themes were identified by the communities around the province as the most significant to Punjabi Canadian history in BC – trans-Pacific journeys, families and homes, community celebrations and commemorations, sawmill experiences and community activism for rights and justice.
This exhibit marked a historic moment for the underrepresented Punjabi Canadian stories in BC to be collectively preserved and shared. ‘Haq’ means rights, and the PCLP is about putting community voices and history in their rightful places. Next to the kiosk in the gallery, Mo ended the event by leading three calls of ‘Inquilab Zindabad!’ – ‘Long live the revolution!’ (in Punjabi) – calling for continuous work to move beyond traditional boundaries and create new paradigms for heritage work.
The Haq & History exhibit, pending funding, will later travel to the communities who helped shape this work.
Event Images:
The kiosk in the Logging area in the museum permanent gallery.
The Haq & History Pocket Gallery display
FTL post
In science fiction, FTL means Faster Than Light. But today I am changing the acronym to suit my needs – Fast Tiny Lizard. On June 19th, 2019, Cathy Judd caught a lizard in an industrial area of Vancouver, and reported to Ashlea Veldhoen (Habitat Acquisition Trust) for identification. I got an email from Ashlea stating that a small green coloured lizard had been caught in Vancouver. I could hear Captain Piccard bellowing Red Alert in my nerdy mind, and I replied with questions and an urgent plea: “PLEASE DO NOT LET IT LOOSE.”
Then I asked for pictures.
I was expecting to see a photo of a Common Wall Lizard in any reply – but nope – to my delight and horror, the lizard in the photograph had two well defined stripes and a pale belly with no dark blotches – it was obviously not a Common Wall Lizard.
This little lacertid was an Italian Wall Lizard (Podarcis siculus) – a species known from scattered locations in the United States including a population on Orcas Island, Washington. They apparently have existed on Orcas island for about 12 years now, but had only been reported in 2018.
Dorsal view of the female Podarcis siculus (RBCM 2187, TL = 143 mm, SVL = 59 mm) from Vancouver, British Columbia.
Ventral view of the female Podarcis siculus (RBCM 2187) from Vancouver, British Columbia.
This lizard, a female, based on its light build and weakly developed femoral pores (males have well developed pores along their thighs) is a first for BC, and possibly also for Canada.
This lizard could have carried a clutch of 5 or more eggs. But no others have been seen in the area, so I assume this Italian immigrant was a lone stow-away. How it got here is a complete mystery. Was it a dumped pet? Did it stow away in camping gear used on Orcas Island? Or was it hiding in shipping materials from Italy or one of the colonized locations in the USA? We may never know. Perhaps some enterprising student will want to examine its DNA and see if there is a match for a source population.
Since Italian Wall Lizards are established on Orcas Island in the same climatic zone as southern Vancouver Island and Vancouver, is successful in cooler climates elsewhere in North America, and known to prey on animals as large as shrews, it represents a high risk invader. At least this one is safely preserved in the RBCM collection, and won’t be the matriarch of a second invading Podarcis species.
Missed the Bus post
Some people get angry when they miss a bus. This morning as I crossed Bowker Creek, and saw my bus heading south. It was a cool morning. It had rained overnight. The air smelled of spring. Birds were everywhere. Missing the bus was a good thing.
Even along suburbian streets, you can do a bit of bird watching when you miss a bus. Regulars along my street include: Northern Flicker, Common Raven, Northwestern Crow, American Robin, Bewick’s Wren, Merlin, Spotted Towhee, Chestnut-backed Chickadee, and Anna’s Hummingbird.
A nesting Anna’s Hummingbird – our neighbour’s Holly bush makes pretty safe nesting habitat.
Overhead you can see a range of gulls, Great Blue Herons, Red-tailed Hawks, Turkey Vultures, and Bald Eagles. Mallards and Canada Geese fly over on their way to and from the local golf course water traps, and a pair of Mallards have taken up residence on the neighbour’s lawn.
And it is no surprise we have exotics like the California Quail, House Sparrow, and European Starling.
Violet-green Swallow – always fun to see them overhead.
In summer we get Violet-green Swallows, Barn Swallows, Downy and Hairy Woodpeckers, White-crowned Sparrow, Purple Finches, and the occasional Western Tanager, Wilson’s Snipe and Common Nighthawk. A few years ago over 30 nighthawks cruised the neighbourhood – that was neat. Steller’s Jay, Varied Thrush, Golden-crowned Sparrow, and Dark-eyed Junco are more common in winter.
Missing from this list is owls – there have to be suburban owls in my neighbourhood – especially Barred Owls – I just haven’t seen any.
Great Horned Owl – not in my garden though (the jesses give it away).
This morning I walked smack into the middle of an irruption of Bushtits, Chestnut-backed Chickadees and Golden-crowned Kinglets. It’s hard to be mad at missing a bus when you are surrounded by those tiny energetic birds, each searching for a morning meal. Then an Anna’s Hummingbird chased one crow away, and while another crow flew by with nesting material in its beak. Seconds later, the bus arrived to ruin the fun – everyone on board staring at their smartphones.
I wonder if any of the passengers were checking a birding app? Were they on iBird? Ebird? Or adding a sighting to iNaturalist? I wonder how many know that a smartphone can be used for more than playing Angry Birds.
The diversity of wildlife apps is increasing at a time when global biodiversity is in peril. Some apps like the Sibley’s bird guide on my phone are more for simple identification and to log your life list. Others feed into bigger networks that help science and feed into wildlife management decisions.
Not sure which app is right for you? Read this review of 5 top birding apps.
If you aren’t interested in slogging through a birding app in a phone or tablet, maybe this would be for you – a project proposed for Google Glass – Glass Birds – as for this YouTube video though – anyone can ID a Western Tanager.
I wonder if Glass Birds is refined to ID birds like this Rock Wren? I need the Google Glasses for gulls, some sparrows, fall warblers, flycatchers, and shorebirds, and best of all, I would be a taxonomic cyborg!
We hear on the news about our armed forces personnel helping people overseas during peacekeeping missions. This year we saw our forces helping flood victims and airlifting communities threatened by forest fires here in Canada. But military presence also can help wildlife – yes, wildlife can flourish even on live fire ranges where tanks range far and wide.
A frame from Sebastian Koerner‘s film footage of a wolf pup and a Marten (Marder) in the Munster-Military training area Lüneburger Heide, Germany.
A recent article in the NewScientist detailed how military ranges are benefitting wolves in Europe. Large carnivores tend to fare poorly when in close contact with our species – we shoot them, our highways and industry fragment and destroy their habitat, and we can thank our automotive industry and its infrastructure for the term Road Kill.
On military ranges, the gun fire is routine and predictable and animals get accustomed to the episodic timing of activity. The range itself and the area nearby generally is devoid of people – except for the short periods during training exercises – and the military presence is a huge deterrent for poachers. I took a Wildlife Management course years ago, and the professor said, “Wildlife management is not about controlling wildlife, it is about controlling people.”
Regulation of human activity in military training areas is so effective, wolves seem to prefer testing ranges in Germany – they are more abundant in military training areas than in nearby wildlife management areas. In addition, roads in testing ranges make convenient thoroughfares for large carnivores and have far less traffic compared to highways near parks.
While reading this NewScientist article I had a flashback to my M.Sc. supervisor, Ken Stewart and his recollection of a misty morning searching for snakes around the Canadian Forces Base in Shilo, Manitoba. The testing range near CFB Shilo is adjacent to the Sprucewoods region and what was once Lake Agassiz beachfront, is now undulating terrain and perfect for training against hidden targets. For tank crews, this sandy, gravelly substrate reduces the risk of ricochets during live-fire exercises and makes it easier to retrieve ordnance. This same sandy habitat also is home to some rarely encountered animals and plants in that province.
The Plains Hognose Snake (Heterodon nasicus); photo by Dean Hester.
Ken described his morning, bushwhacking through a forested area adjacent to the base and how the conifers reminded him of the Ardennes Forest and the Battle of the Bulge. He imagined he morning’s sub-silence shattered by the clanking tracks and diesel engine of a Tiger Tank, and said, “All I needed was Lili Marlène playing on an old turntable to complete the scene.” But when Ken was there, it would have been a Leopard, not a Tiger ensuring that all but the most intrepid herpetologists were kept well-clear.
Leopard 1 Main Battle Tank; photo by Sergeant Dennis Power, Army News-Shilo. ©2008 DND/MDN Canada
Essentially, this pocket of land within the ranges of the Northern Prairie Skink (Plestiodon septentrionalis) and Plains Hognose Snake (Heterodon nasicus) is protected by our armed forces and right next door to Spruce Woods Provincial Park. Ken’s story dates back years before he and I met – and so the concept of military ranges as critical shelter for wildlife is certainly not new. CFB Shilo, recently saluted for its work on wildlife conservation, has a Base Biologist who oversees natural resource management, gives tours of the sand dunes, and facilitates research in this restricted area. Other bases like CFB Suffield provide grassland habitat for a myriad of species including Burrowing Owls, Sprague’s Pipit, Pronghorn and Ord’s Kangaroo Rat. CFB Gagetown is working to protect one of nature’s tanks – the endangered Wood Turtle.
Sandy blow-out areas and mixed woods habitat protected by CFB Shilo, image courtesy of the Manitoba Association of Plant Biologists.
Since the concept of military ranges as shelter for wildlife is not new, the NewScientist story on wolves serves to reinforce the fact that human activity now is the primary factor influencing nature – as also stated in Bill McKibben’s book, The End of Nature. If we restrict human activity, nature thrives. Since wolves in BC capitalize on human activity and use pipelines, roads and seismic lines cut during oil and gas exploration, and well-packed snowmobile trails as easy avenues to track Caribou, you can bet BC’s wolves also could adapt to military ranges as their relatives do in Germany. The list of species protected on Department of National Defense (DND) land is extensive and it is good to see that in areas where soldiers practice and you’d expect disruption and devastation – the opposite is true – human activity is restricted, and life adapts to, and is protected by our armed forces.
Branching Out post
I recently spent an enjoyable day hunting Common Wall Lizards to help Camosun College students with a research project. Lizards were easily caught with nooses, by hand, and using elastic bands. These lizards are to be used in a diet study to see whether there is any pattern between historic and current arthropod diversity in pitfall trap samples, and to determine what lizards are selecting from the available invertebrates.
We sampled at Haliburton Farm in Saanich, here on Vancouver Island. Lizards were everywhere – and that is no exaggeration. Every few steps would cause one or more lizards to skitter way into the forest of potted plants and garden veggies growing at the farm.
My Google Earth Map totally under estimates the number of lizards because I couldn’t map the location of each one. There were hundreds of lizards in each section of the farm. Adults were predominant in the heavily modified areas, and yearlings seemed to be occupying peripheral areas that were almost semi natural – young ones likely avoided the main farm to avoid cannibalism.
The tree and a closer view of the knot-hole where the Wall Lizard sought refuge.
One lizard stuck out in my memory – because it was trying to shed the “wall lizard” stereotype by living in a tree. I spotted an adult male well up a tree – and as I approached, it bolted into a knot-hole. The knot-hole led to a significant cavity inside – I used a long dry grass stem to get an idea how large the cavity was. It was at least 20 cm long, plenty of room for an adult Wall Lizard. Years ago Richard Hebda noted that Common Wall Lizards had started to occupy grassy habitat as well as the typical more solid habitat. This lizard seemed more interested in becoming a Tree Lizard – sorry Podarcis, you can change habitat, but not your taxonomy. Luckily Urosaurus ornatus does not live here and won’t have to deal with this arboreally inclined invader.
A tale of two tails post
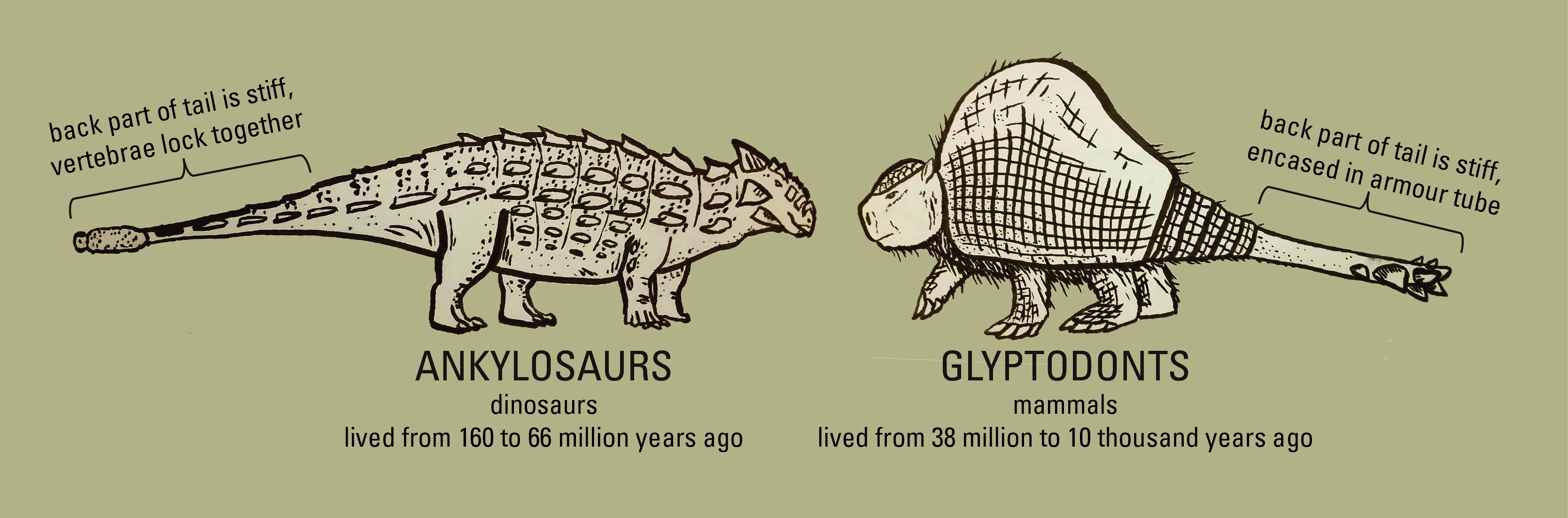
The armoured glyptodonts and ankylosaurs are one of my favourite examples of convergent evolution, the evolutionary phenomenon in which distantly related animals evolve similar structures or body shapes. Ankylosaurs are the armoured dinosaurs covered in bony plates called osteoderms, and are one of my favourite groups of dinosaurs. Glyptodonts, on the other hand, are mammals – they’re an exinct group of giant, herbivorous armadillos that disappeared about 10 000 years ago. The last time glyptodonts and ankylosaurs shared a common ancestor – a great-great-great-great-grandparent, if you will – was over 300 million years ago, but these two groups of animals evolved similar anatomical features. Most unusually, both ankylosaurs and glyptodonts evolved weaponized, sledgehammer-like tails.
In this study, I worked with my colleague (and former postdoctoral supervisor) Dr. Lindsay Zanno at the North Carolina Museum of Natural Sciences to figure out whether or not ankylosaurs and glyptodonts had followed similar evolutionary trajectories when evolving their unusual tail weaponry. Lindsay and I have previously worked on understanding the evolution of bony tail weapons across amniotes (turtles, lizards, crocodilians, birds, mammals, and their extinct relatives) and found that certain anatomical features like armour, large body size, and a stiff backbone were correlated with bony tail weaponry. For our new study, we dug deeper into the anatomy of ankylosaurs and glyptodonts. We wanted to know whether or not ankylosaurs and glyptodonts evolved some of their distinct features in the same way – did certain features evolve before others in both groups? By studying fossils in museums around the world, we were able to map features onto the family trees for ankylosaurs and glyptodonts and see at what points different features first evolved. It turned out that, despite a few differences, the overall pattern was the same: both groups evolved armour, large body size, and stiff backs before weaponizing their tails, and tails became stiff before the tip of the tail was expanded.
What does this similar pattern tell us about how or why tail clubs evolved in glyptodonts and ankylosaurs? When we see similar adaptations in unrelated species, it tells us that there might only be a few good solutions to the challenges that nature throws our way, or in other words, similar features evolve when species are faced with similar selective pressures. In this case,
Lindsay and I speculate that a heavy, expanded tail tip might not be able to evolve unless the tail is already modified to support the extra weight. Similarly, swinging a heavy tail club around might be easier if you have a stiff backbone to help brace against impacts. And lastly, the rarity of species with tail clubs in the fossil record also suggests that tail clubs aren’t easy structures to evolve, and might only be able to evolve when a lot of other anatomical features (like armour) are already in place.
Funding for this research was generously provided by NSERC, the North Carolina Museum of Natural Sciences, and the Jurassic Foundation.
Arbour VM, Zanno LE. 2019. Tail weaponry in ankylosaurs and glyptodonts: an example of a rare but strongly convergent phenotype. The Anatomical Record.
Abstract: The unusual clubbed tails of glyptodonts among mammals and ankylosaurines among dinosaurs most likely functioned as weapons of intraspecific combat or interspecific defense and are characterized by stiffening of the distal tail and, in some taxa, expansion of the distal tail tip. Although similarities in tail weaponry have been noted as a potential example of convergent evolution, this hypothesis has not been tested quantitatively, particularly with metrics that can distinguish convergence from long‐term stasis, assess the relative strength of convergence, and identify potential constraints in the appearance of traits during the stepwise, independent evolution of these structures. Using recently developed metrics of convergence within a phylomorphospace framework, we document that convergence accounts for over 80% of the morphological evolution in traits associated with tail weaponry in ankylosaurs and glyptodonts. In addition, we find that ankylosaurs and glyptodonts shared an independently derived, yet constrained progression of traits correlated with the presence of a tail club, including stiffening of the distal tail as a precedent to expansion of the tail tip in both clades. Despite differences in the anatomical construction of the tail club linked to lineage‐specific historical contingency, these lineages experienced pronounced, quantifiable convergent evolution, supporting hypotheses of functional constraints and shared selective pressures on the evolution of these distinctive weapons.
Last summer my wife and I bought a new car – it is less than a year old and has already transported quite an assemblage of BC species (Southern Resident Killer Whale foetus, Mule Deer, River Otter, Red Fox, Northern Alligator Lizard, Common Wall Lizard, Commander Skate, and 49 species of birds – including the museum’s first Brown Booby). The most recent passenger was a 1.2 meter Shortfin Mako Shark (Isurus oxyrhinchus) which easily fit into the back of a 2018 Nissan Leaf. Chalk up another reason why electric cars are awesome.
The Mako Shark (wrapped in plastic) arrives at the RBCM loading bay.
As far as I know, this is the second Shortfin Mako Shark specimen from BC waters. The first specimen, from 185 nautical miles west of Cape St. James (Haida Gwaii), was made into a taxidermy mount and only a few of its teeth were deposited in the Royal BC Museum collection (993-00039-001). You have to wonder how often they range this far north?
The mako shark thawed and ready for a long soak in formaldehyde.
This new mako, found September 27, 2016 on shore in Florencia Bay, Pacific Rim National Park Reserve is almost perfect. It had been studied by Jackie King (Fisheries and Oceans Canada), tissue samples were taken, and then was shuttled to the Institute of Ocean Sciences (IOS) in Sidney. I picked up the fish at IOS and kept it frozen until I had the time to prepare the shark for the Royal BC Museum collection. Mako sharks are most streamlined representatives of the Family Lamnidae, the same family containing the Great White Shark. It was a thrill to see this amazing fish up close. Its only damage came from scavengers – the left eye is missing, and something – a wolf(?) – had ripped at the gills on the left side.
Tooth rows are easy to see in the jaws of this Shortfin Mako.
The teeth are amazing – and let’s face it – this is what most people want to see on a shark. But have a look at the tail! Without an efficient tail – the teeth would have nothing to bite. Mako sharks are amazingly fast and almost appear nervous when they are swimming – they are certainly the Formula-e cars or jet fighters of the shark world.
The base of the tail on our new mako shark.
Makos have a lateral keel at the base of the tail which allows the fish to efficiently oscillate its tail fin from side to side. In lateral view the base of the tail is narrow – in dorsal / ventral view – the tail base is broad. Salmon Sharks (Lamna ditropis), Porbeagles (Lamna nasus) and Great White Sharks (Carcharodon carcharias) have this same feature – it is all about efficient locomotion – hydrodynamics which submarine designers envy. Even the Ninespine Stickleback (Pungitius2) has this basic tail structure – but on a far smaller fish. Evolution is awesome.
Enough fish worship – back to the task at hand. Preservation of a large fish. You have to make sure the internal organs and muscles fix – and since formaldehyde takes time to infiltrate tissues – you inject 10% formaldehyde deep into the muscles to make sure the specimen fixes from the outside in, and inside out.
If the specimen does not fix fairly rapidly – then decay of the tissues begins. The specimen degrades and gas is produced. A gas-filled specimen displaces fluid and can result in a bit of a mess in the lab. When I was a student, we put a sizable sample of suckers in a vat of formaldehyde, closed the lid, and then left them to fix. Oily suckers are always a challenge to fix, and these were no exception. They bloated over night and displaced formaldehyde – which spilled out of the vat. The spill was large enough to draw the attention of the University of Manitoba’s Workplace Health And Safety team. Ooops.
Reptiles also can be tricky to fix – their skin slows the uptake of formaldehyde. As a dewy-eyed student I was keen to check out all the specimens in the vertebrates lab – and was particularly happy to find a forgotten jar with dark brown glass – a mystery. I had to know what was inside. When I reached in and grabbed the snake – it simply fell apart – ribs straining through my fingers. The mouth and cloaca allowed formaldehyde to enter and so the snake’s head and tail preserved well. Its body though, had rotted from the inside out and was mush.
The rattle from the rotten Pacific Rattlesnake (Crotalus oreganus).
I now use a needle to perforate reptile legs and tails to make sure formaldehyde infiltrates everywhere. I also inject 10% formaldehyde into the body cavity to make sure the internal organs of reptiles fix rapidly.
This mako shark was no different – I injected about 500 ml formaldehyde into the body cavity to make sure the internal organs fixed well – then left for the weekend. After a day in formaldehyde, the body already was rubbery and well on its way to making a decent specimen (yes I came to work on a Saturday to check on my precious). It also was not floating – that is a really good sign that the specimen is fixing well and not filling with decay gases.
The mako shark after a day in formaldehyde.
Once the mako shark is fixed (maybe three weeks in formaldehyde just to be sure), then it will get a rinse in water for a few days, and will go into a vat of alcohol for permanent storage. Alcohol is far easier on the eyes and nose than formaldehyde. Ethanol or Isopropanol are our preservatives of choice. Call me crazy, but I am guessing this shark will be a popular item during museum collection tours, so it better be stored in a manner that is fairly safe for visitors. I may as well get a few more larger fishes preserved while the formaldehyde vat is fresh – next up is a 1 meter Blue Shark (Prionace glauca) and a similarly sized Pacific Sleeper Shark (Somniosus pacificus).
Mighty Mouth post
Museums contain the commonplace, normal, typical specimens as well as the specimens we call TYPES which serve as the golden standard when doing systematics research. But the real attention goes to the oddities – they seem to naturally draw your eyes away from all other specimens. Leucistic birds, albinos, a marmot with overgrown incisors, an Orca with nasty dental issues, an Orca with spinal deformity – these are the specimens that get the WOW vote on collections tours.
Albino Starlings in the Royal BC Museum Ornithology collection – abnormal specimens certainly do catch your eye.
A year or so ago we were clearing out an area we referred to as Room 17 (our version of Area 51), and found jaw fragments from a Sperm Whale mixed with the bones of other whales. Sperm Whales have teeth along the lower jaw but no teeth along the upper jaw and Sperm Whale jaws are long and straight. It does not take a scientific eye to notice what is wrong with these jaws.
The section of Sperm Whale jaw in the Royal BC Museum collection.
Both dentary bones are hooked to the left and it looked like the teeth were fairly normal with decent sized sockets. We have no idea what the upper lip looked like – but I assume these jaws just hooked out of alignment and hung out to the side of the animal. What a drag that would have been. The jaws are large – so this animal was able to feed – the teeth towards the back of the jaw probably functioned normally and it certainly could have performed suction feeding to catch fishes and cephalopods.
Strangely enough, there is no information with these jaws to say when and where the animal was caught. Whaling here in BC ended within my lifetime (including the live capture of Orcas as a form of whaling – some would say jailing) – so I can only assume this jaw was collected pre-1970s when whaling stations were still actively processing Sperm Whales.
Sperm Whales with normal straight jaws (Image A-09221 (top) and Image A-09220 (bottom) courtesy of the Royal BC Museum and Archives).
Jaw deformities are not that rare in Sperm Whales – there are several reports published and some of the deformities are shocking – some are stubby, others in a tight spiral like a conispiral snail shell (see: Murie 1865, Thomson 1867, Nasu 1958, Spaul 1964, and Nakamura 1968).
This specimen is not cataloged in the Royal BC Museum Mammalogy collection, there is no record in our database, and no mention in the museum’s annual reports. Perhaps whaling records will mention this animal – I can’t imagine this whale was processed in the ‘fishery’ and the set of jaws saved with no comment made of the deformity. Time to go CSI on this dentary record.
To dig deeper:
MURIE, J. 1865. On deformity of the lower jaw in the cachalot (Physeter macrocephalus, Linn.). Proceedings of the Zoological Society of London, 1865: 396-396.
NAKAMURA, K. 1968. Studies on the sperm whale with deformed lower jaw with special reference to its feeding. Bulletin of the Kanagawa Prefecture Museum of Natural History, 1: 13-27.
NASU, K. 1958. Deformed lower jaw of sperm whale. Scientific Reports of the Whales Research Institute, 13: 211-212.
SPAUL, E. A. 1964. Deformity in the lower jaw of the sperm whale (Physeter catodon). Proceedings of the Zoological Society of London, 142: 391-395.
THOMSON, J. H. 1867. Letter relating to the occasional deformity of the lower jaw of the sperm whale. Proceedings of the Zoological Society of London, 1867: 246-247.
Frost-Free Footwear post
When reptiles and amphibians take shelter from the cold, they seek refuge above freezing, but not too warm – maybe 2 to 4°C. If it is too cold, tissues freeze and for most animals, this is fatal. Death comes from ice crystal growth which essentially shreds body cells at a microscopic level. Some animals like the Wood Frog (Rana sylvatica) are freeze–tolerant, and consequently are our northern most ‘herpetile’ along the coast of the Arctic Ocean/Beaufort Sea.
Wood Frog photographed in a ditch near Winnipeg.
If the refuge is too warm, the animal’s metabolism burns stored fat and the animal loses weight. My father had a pet Hermann’s Tortoise (Testudo hermanni) when he was a child – and in winter, his parents put the tortoise in a box, surrounded it with hay, and placed it in the boiler room to hibernate. The boiler room was too warm for hibernation – the tortoise starved or died of dehydration. Consequently, my grandparents bought a new tortoise each year after burying its ‘hibernating’ predecessor. I am not shore how many tortoises they went through.
Hermann’s Tortoise image from Wikipedia.
On March 5th (2019) I received a set of photos of a frozen adult Wall Lizard found by John Hunter, a Colwood resident. It appeared that the cold, frosty nights of Late February and Early March 2019 had claimed at least one lizard life. The lizard had taken refuge in John’s gardening shoes. Its body was placed on a rock in the garden – presumably nature would deal with the remains. March 6th – the lizard awoke and ran off.
Resurrection? No. The Common Wall Lizard (Podarcis muralis), like the Wood Frog, has a physiological ace up its sleeve. It can freeze up to 28% of its body water and still survive. As long as the cold snap is not too long or too severe, they usually survive without trouble. The mild winters of southern Vancouver Island were almost tailor-made for these invaders.
However, shoes obviously were not ideal shelter from the cold. Shoes keep our feet warm because our feet produce heat – the shoe only slows heat loss to the environment. Has anyone ever said to you, “Here, this blanket will warm you up.” Truth is, a blanket doesn’t provide heat, only slows heat loss – just like winter boots. With no source of heat, and with the open cuff/collar, footwear would act more like a sci-fi cryo-tube than a cozy refuge. At best the shoes shielded the lizard from scavengers.
I left my gardening boots outside last week – they were a bit too muddy to bring indoors. But since lizards are still about 60 meters north of my garden – I don’t think I will find any lost souls lining the insole.
Odonata of Canada post
Robert A. Cannings¹
1 Royal British Columbia Museum, 675 Belleville St, Victoria, BC, V8W 9W2, Canada
Abstract
Since Corbet’s thorough 1979 overview of Canadian Odonata, hundreds of regional works on taxonomy, faunistics, distribution, life history, ecology and behaviour have been written. Canada records 214 species of Odonata, an increase of 20 since the 1979 assessment. Estimates of unrecorded species are small; this reflects the well-known nature of the fauna. A major impetus for surveys and analyses of the status of species is the work of the Committee on the Status of Endangered Wildlife in Canada which provides a scientifically sound classification of wildlife species potentially at risk. As of 2017, six species have been designated “Endangered” and two “Special Concern” (only five of which are officially listed under the Federal Species at Risk Act (SARA)). The Order provides a good example of molecular bar-coding effort in insects, as many well-accepted morphological species in Canada have been bar-coded to some degree. However, more bar-coding of accurately identified specimens of many species is still required, especially in most of the larger families, which have less than 70% of their species bar-coded. Corbet noted that the larvae of 15 Canadian species were unknown, but almost all larvae are now well, or cursorily, described. Extensive surveys have greatly improved our understanding of species’ geographical distributions, habitat requirements and conservation status but more research is required to better define occurrence, abundance and biological details for almost all species.
Keywords
barcoding, biodiversity assessment, Biota of Canada, climate change, identification, Odonata, species at risk
Read full article
Diptera of Canada post
Jade Savage¹, Art Borkent³, Fenja Brodo¹¹, Jeffey M. Cumming², Gregory Curler⁴, Douglas C. Currie⁵, Jeremy R. deWaard⁶, Joel F. Gibson³, Martin Hauser⁷, Louis Laplante⁸, Owen Lonsdale², Stephen A. Marshall⁹, James E. O’Hara², Bradley J. Sinclair¹⁰, Jeffey H. Skevington²
1 Bishop’s University, Sherbrooke, Quebec, Canada 2 Agriculture and Agri-Food Canada, Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Ontario, Canada 3 Royal British Columbia Museum, Victoria, British Columbia, Canada 4 Mississippi Entomological Museum, Mississippi State University, Starksville, Mississippi, USA 5 Royal Ontario Museum, Toronto, Ontario, Canada 6 Centre for Biodiversity Genomics, University of Guelph, Guelph, Ontario, Canada 7 California Department of Food and Agriculture, Sacramento, California, USA 8 Unaffiated, Montreal, Quebec, Canada 9 University of Guelph, Guelph, Ontario, Canada 10 Canadian Food Inspection Agency, Ottawa, Ontario, Canada 11 Canadian Museum of Nature, Ottawa, Ontario, Canada
Abstract
The Canadian Diptera fauna is updated. Numbers of species currently known from Canada, total Bar-code Index Numbers (BINs), and estimated numbers of undescribed or unrecorded species are provided for each family. An overview of recent changes in the systematics and Canadian faunistics of major groups is provided as well as some general information on biology and life history. A total of 116 families and 9620 described species of Canadian Diptera are reported, representing more than a 36% increase in species numbers since the last comparable assessment by JF McAlpine et al. (1979). Almost 30,000 BINs have so far been obtained from flies in Canada. Estimates of additional number of species remaining to be documented in the country range from 5200 to 20,400.
Keywords
biodiversity assessment, Biota of Canada, Diptera, flies, systematics
Read full article
Mecoptera of Canada post
David C.A. Blades¹
1 Research Associate, Royal British Columbia Museum, 675 Belleville St, Victoria, BC, V8W 9W2, Canada
Abstract
The Mecoptera are represented in Canada by 25 extant species in four families, an increase of three species since the prior assessment in 1979. An additional 18 or more species and one family are expected to occur in Canada based on distributional records, recent collections and DNA analyses. The Bar-code of Life Data System currently lists 24 Bar-code Index Numbers for Canadian Mecoptera. There are nine species of fossil Mecoptera known from Canada
Keywords
biodiversity assessment, Biota of Canada, Mecoptera, scorpionfly
Read full article
James Miskelly¹, Steven M. Paiero²
1 Royal British Columbia Museum, 675 Belleville St., Victoria, British Columbia, V8W 9W2, Canada 2 School of Environmental Sciences, 50 Stone Rd. East, University of Guelph, Guelph, Ontario, N1G 2W1, Canada
Abstract
In the last 40 years, the number of species in the orthopteroid orders has increased by ~10% from that known in 1979. The largest order, the Orthoptera, has increased from 205 to 235 species known in Canada. The number of Blattodea has increased from 14 to 18 species, while Dermaptera has increased from 5 to 6 species. The number of species of Mantodea (3) and Phasmida (1) known in Canada have remained unchanged. Most new species records reported in Canada since 1979 have resulted from new collections along the periphery of the range of more widespread species. Some species reported since 1979 are recent introductions to Canada, including species restricted to homes or other heated buildings. The taxonomy of these orders has also changed, with only the Dermaptera having maintained its order definition since the 1979 treatment. Additional orthopteroid species are likely to occur in Canada, particularly in the orders Orthoptera and Blattodea. DNA bar-codes are available for more than 60% of the species known to occur in Canada
Keywords
biodiversity assessment, Biota of Canada, Blattodea, cockroaches, crickets, Dermaptera, earwigs, grasshoppers, katydids, mantids, Mantodea, Orthoptera, Phasmida, stick insects, termites
Read full article
David W. Langor¹
1 Natural Resources Canada, Canadian Forest Service, 5320 – 122 St. NW, Edmonton, Alberta, T6H 3S5, Canada
Abstract
Based on data presented in 29 papers published in the Biota of Canada Special Issue of ZooKeys and data provided herein about Zygentoma, more than 44,100 described species of terrestrial arthropods (Arachnida, Myriapoda, Insecta, Entognatha) are now known from Canada. This represents more than a 34% increase in the number of described species reported 40 years ago (Danks 1979a). The most speciose groups are Diptera (9620 spp.), Hymenoptera (8757), and Coleoptera (8302). Less than 5% of the fauna has a natural Holarctic distribution and an additional 5.1% are non-native species. A conservatively estimated 27,000–42,600 additional species are expected to be eventually discovered in Canada, meaning that the total national species richness is ca. 71,100–86,700 and that currently 51–62% of the fauna is known. Of the most diverse groups, those that are least known, in terms of percent of the Canadian fauna that is documented, are Acari (31%), Thsanoptera (37%), Hymenoptera (46%), and Diptera (32–65%). All groups but Pauropoda have DNA barcodes based on Canadian material. More than 75,600 Barcode Index Numbers have been assigned to Canadian terrestrial arthropods, 63.5% of which are Diptera and Hymenoptera. Much work remains before the Canadian fauna is fully documented, and this will require decades to achieve. In particular, greater and more strategic investment in surveys and taxonomy (including DNA barcoding) is needed to adequately document the fauna.
Keywords
Arachnida, biodiversity assessment, Biota of Canada, checklists, Entognatha, Hexapoda, Insecta, Myriapoda, surveys, taxonomy, Zygentoma
Read full article
Neuroptera of Canada post
David C.A. Blades¹
1 Research Associate, Royal British Columbia Museum, 675 Belleville St, Victoria, BC, V8W 9W2, Canada
Abstract
The Neuroptera of Canada consists of 101 extant species, an increase of 26 (35%) since the previous assessment of the fauna in 1979. More than 48 additional species are believed to occur in Canada based largely on recent DNA evidence and new distribution records. The Bar-code Of Life Data System (BOLD) currently includes 141 Bar-code Index Numbers (BINs) for Canadian Neuroptera. Canadian fossils have thus far yielded 15 species in three families of Neuroptera.
Keywords
antlion, aphidlion, biodiversity assessment, Biota of Canada, lacewing, mantidfly, Neuroptera, owlfly
Read full article
Hemiptera of Canada post
Robert G. Foottit¹, H. Eric L. Maw¹, Joel H. Kits¹, Geoffey G. E. Scudder²
1 Agriculture and Agri-Food Canada, Ottawa Research and Development Centre and Canadian National Collection of Insects, Arachnids and Nematodes, K. W. Neatby Bldg., 960 Carling Ave., Ottawa, Ontario, K1A 0C6, Canada 2 Department of Zoology and Biodiversity Research Centre, University of British Columbia, 6270 University Boulevard, Vancouver, British Columbia, V6T 1Z4, Canada
Abstract
Th Canadian Hemiptera (Sternorrhyncha, Auchenorrhyncha, and Heteroptera) fauna is reviewed, which currently comprises 4011 species, including 405 non-native species. DNA bar-codes available for Canadian specimens are represented by 3275 BINs. Th analysis was based on the most recent checklist of Hemiptera in Canada (Maw et al. 2000) and subsequent collection records, literature records and compilation of DNA bar-code data. It is estimated that almost 600 additional species remain to be discovered among Canadian Hemiptera.
Keywords
Barcode Index Number (BIN), biodiversity assessment, Biota of Canada, DNA barcodes, Hemiptera, true bugs
Read full article
David C.A. Blades¹
1 Research Associate, Royal British Columbia Museum, 675 Belleville St, Victoria, BC, V8W 9W2, Canada
Abstract
There are eight species in two families of Raphidioptera known from Canada, an increase of one species since the prior assessment in 1979. Another four species are likely to occur in Canada based on DNA evidence and distributional records. The Bar-code of Life Data System currently lists ten Bar-code Index Numbers for Canadian Raphidioptera.
Keywords
biodiversity assessment, Biota of Canada, Raphidioptera, snakeflies
Read full article
Araneae of Canada post
Robb Bennett¹, Gergin Blagoev², Claudia Copley¹
1 Department of Entomology, Natural History Section, Royal British Columbia Museum, 675 Belleville Street, Victoria, British Columbia, V8W 9W2, Canada 2 Centre for Biodiversity Genomics, University of Guelph, 579 Gordon Street, Guelph, Ontario, N1G 2W1, Canada
Abstract
In 1979 nearly 1400 spider species in 32 families either had been recorded (1249) or were believed to occur (~140) in Canada. Twenty years later, although significant progress had been made in survey efforts in some regions, Canada’s spider inventory had only increased by approximately 7% to roughly 1500 species known or expected to occur. Th family count had increased to 38 but only two additions were truly novel (fie family additions and one family deletion were the result of advances in family-level systematics). The first comprehensive taxonomic checklist of Canadian spider species was published in 2010 documenting the regional distributions of 1376 species representing 42 families (three novel since 1999). From 2010 through 2017 new national records steadily accumulated resulting in the current (2018) Canadian inventory of 1477 species classified in 45 families (one novel since 2010). Although there has been close to a 20% increase in the number of spider species recorded in Canada since 1979, much greater increases have occurred in some of the regional species checklists, indicating increasing knowledge of the regional distribution of species previously recorded elsewhere in Canada. For example the regional checklists for Newfoundland, British Columbia, and Prince Edward Island have increased by 69%, 339%, and 520%, respectively. The national and regional increases reflect significant advances in the fist two decades of the 21 st Century in spider faunistics research in previously under-sampled habitats and regions and the development of molecular techniques and consequent bar-coding of spiders. Of the 1477 species recorded in Canada, 92% have been successfully DNA bar-coded resulting in 1623 unique Bar-code Index Numbers (BINs). At least 25 of the BINs are associated with relatively easily distinguished but undescribed morpho-species. Th majority, however, appear to indicate the existence of many cryptic species within Canada’s known spider fauna. Thse data, coupled with the fact that novel Canadian or even Nearctic spider species records (including of undescribed species) continue to accumulate annually (especially in habitat-diverse regions such as British Columbia), suggest that Canada’s tally of spider species may approach or even exceed 1800.
Keywords
Araneae, BINs, biodiversity assessment, Biota of Canada, checklist, classification, DNA barcoding, faunistics, spiders
See full article
I’m thrilled to announce the publication of my first book, Zuul: Life of an Armoured Dinosaur! Co-authored with my colleague Dr. David Evans (Temerty Chair of Vertebrate Paleontology at the Royal Ontario Museum) and published through the ROM Press, this book explores the discovery of a spectacular armoured dinosaur skeleton and what it’s revealing about the evolution and biology of these unusual dinosaurs.
For over a decade I’ve been interested in the palaeobiology of ankylosaurs, a fascinating group of extinct dinosaurs with a spiky, armoured appearance. I’ve studied how they used their unusual tail clubs and how those tail clubs evolved, how different species around the world are related to each other, and how those species changed over time. In 2016, when I joined David’s lab at the Royal Ontario Museum and University of Toronto as an NSERC postdoctoral fellow, I had the incredible opportunity to study a brand new dinosaur known from a nearly complete, exceptionally well-preserved skeleton. David and I named this new dinosaur Zuul crurivastator in a May 2017 paper published in Royal Society Open Science. The genus name, Zuul, is after the Ghostbusters monster of the same name, and the species name, crurivastator, means ‘destroyer of shins’ in Latin, in reference to its sledgehammer-like tail club.
In Zuul: Life of an Armoured Dinosaur, David and I pull together our recent and ongoing research on Zuul, my experience studying the biology of armoured dinosaurs as a whole, and David’s work on the dinosaurs of southern Alberta and Montana. We share how Zuul’s skeleton was discovered and excavated in the badlands of Montana and how it made its way to Toronto. We describe how it was named and how it fits into the bigger family tree of ankylosaurs, and what this new specimen is teaching us about ankylosaur armour and weapons. We even get to share what we know about Zuul’s broader ecosystem: the remains of many different species of plants and animals were found alongside Zuul’s skeleton, allowing us to understand Zuul’s friends, foes, and food.
Throughout the book we’ve been able to feature beautiful photographs of this amazing specimen, brand new illustrations by world-renowned palaeoartists Danielle Dufault and Julius Csotonyi, and behind-the-scenes peeks at ongoing scientific research on Zuul. It’s been a blast getting to study this wonderful specimen and work with such a talented team of fossil preparators, artists, exhibit developers, scientists, and the ROM publishing team to bring this book to press. I hope you’ll get a chance to appreciate the beauty and intrigue of these dinosaurs as much as I do!
Signed copies of Zuul: Life of an Armoured Dinosaur are available in the Royal Museum Shop now!

Zuul: Life of an Armoured Dinosaur
Victoria Arbour and David Evans
ROM Press, 2018, 9” x 12”, 96 pages, hard cover
Lettuce Travel post
Lettuce is shipped to Canada regularly. Plastic-wrapped-produce crosses our border every day – it is inspected and then it goes to grocery stores across the province. The lettuce then gets purchased, bagged and taken home – sometimes for sandwiches, salads, or maybe for juicing.
Green goodness at a local grocery store.
This November 27th, a bag of leafy goodness was opened after crossing the international border with a stowaway – a small frog in lettuce from California. It emerged – and was taken to the local SPCA. From there it was sent to me at the Royal BC Museum for identification.
The stowaway was sent to me in a container filled with damp moss.
On first glance this refugee looks like our Pacific Chorus Frog (Pseudacris regilla) which ranges south of BC to California. The taxonomy of the Pacific Chorus Frog is quite contentious though. Historically only one species was defined – P. regilla. In recent years, mitochondrial DNA suggested three species exist in California in what was once a wide-ranging Pacific Chorus Frog. Based on mtDNA, our Pacific Chorus Frog was thought to only range into extreme northwestern California. To the south, the Sierran Chorus Frog (P. sierrae) ranged across central California, and Baja California Chorus Frogs (P. hypochondriaca) were scattered across southern third of that state. If that wasn’t enough to upset a frog’s personal identity, work in 2016 placed the Pacific Chorus Frogs in a new genus Hyliola. Then in 2017, after referring back to a 2014 analysis of nuclear DNA, the three species were once again lumped into Pseudacris regilla. Or is it Hyliola? I bet the frog is confused too.
The range of the three chorus frog species based on mtDNA, from: http://www.californiaherps.com/frogs/maps/pregillamap3species3.jpg
Call me lazy, but if they are all lumped into one species – P. regilla – that makes my life easier. If the Pacific Chorus Frog was split into three species, then either I’d need to take a tissue sample to get an identification (and the frog would not enjoy that), or I’d need to know exactly where the lettuce came from. Odds are grocery records are pretty tight in this era of E. coli-tainted tracheophytes, but I have some doubt we’d ever know exactly where a given bag of lettuce originated.
A Pacific Chorus Frog from just north of the Nighthawk border crossing in the Okanagan.
Let’s just assume we are lumping all the Pacific coast Pseudacris into one species – then this refugee regilla is the same species as our Chorus frogs in BC. If this is the same species, can I just let it go? No way. It is genetically distinct since it comes from so far away, and there always is the risk of disease transmissions posed by exotic frogs. At least this Californian frog didn’t come from a pet shop where it could encounter a range of other exotic frogs and their diseases.
To be honest, I am really impressed that the frog was contained in the first place – people have a habit of releasing stowaways rather than turning them in for examination. Years ago a couple returned home from Mexico and found a red and black snake in their luggage. The snake didn’t seem well, but they released it somewhere in Metchosin. Presumably that snake died, but if it had been a gravid female, it could have deposited 7-10 (or more) eggs, and we’d have an instant population. What species had infiltrated their luggage? I have no idea – it could well have been venomous. When I was an undergrad student, a red and black snake appeared in the pet trade in Winnipeg – it was labeled Honduran Milk Snake and looked like this. I assumed it was harmless based on the old rhyme:
Red-on-Black, Safe for Jack.
Red-on-Yellow, Kill a fellow.
I was wrong – the snake in the pet shop was rear-fanged and bit me. It was my first (and currently only) venomous snake bite. Bottom line is: Better to be safe than sorry. And as a member of IMISWG (Inter-Ministry Invasive Species Working Group) we always say that it is better to not release something, than try to clear out exotic species later. Turn in stowaways to your local SPCA or Natural Resource Officers. It is safer for the environment. Frogs obviously are harmless, but if you think you have something dangerous in your groceries – an Eyelash Viper in a bunch of bananas or a Brown Widow Spider in your Californian cauliflower – call your local Natural Resource office and arrange for a professional to remove the offending animal.
Above all else, don’t let it loose.
Fieldwork 2018
This year’s fieldwork was our 17th in the alpine of the northern B.C. We made collections from six mountains. The area is so vast and remote and access is difficult, thus few if any biological inventories have been undertaken in many large areas. Many peaks and lakes have no names; in fact there are no names on entire mountain massifs. I feel like we are to some degree just ‘scratching the surface’ of what is out there.
We again worked together with the insect and spider experts at the museum. And we followed our typical approach of setting up camp for 2-3 days and collecting specimens of every species we encounter, being intentional to reach as many different habitats as possible.
Typical day of hiking (dotted line) and collecting, striving to reach as many habitats as possible. Upper part of figure documents elevation changes during the day. Three of the four plants in this illustration were encountered only once, highlighting the importance of having several botanists in the field and covering as much ground as possible.
At one mountain, Mt. Whitford, we were joined by two staff and a contract photographer of the Yellowstone to Yukon Conservation Initiative https://y2y.net/about-us and their guest freelance journalist who produces pieces for both CBC and NPR. At a second mountain, south of Tumbler Ridge, we were joined by two staff members of the Tumbler Ridge Geopark, http://tumblerridgegeopark.ca/. What is a Geopark? According to their website “A UNESCO Global Geopark is an area recognized as having internationally significant geological heritage.” These groups are all interested in knowing as much as possible about the biota of these areas and we will share everything we learn with them.
As we have in the past, we contacted the local indigenous groups and informed them of our work and will provide them species lists when the identifications are complete.
We are often asked if we notice any of the effects of climate change during our fieldwork. Treeline is controlled by temperature, not elevation and is highest at the equator – where temperatures are warmer at higher elevations – and becomes lower and lower further north and south. One likely consequence of a warming planet is that forests will advance into the alpine, reducing the available habitat for tundra plants that generally require open, i.e. non-shaded habitats.

Young trees in alpine meadows at 1750 m north of Laurier Pass, Graham-Laurier Provincial Park. Note the dark skies of a storm about to dump
For a number of years I’ve noticed small trees in the alpine and of course wonder if their appearance is related to climate change as a consequence of global warming. I’ve also noticed the absence of dead trees. The absence of dead trees may mean that tree populations in the alpine are relatively young, compared to lower elevation forests where trees have been growing and dying for thousands of years.
Two hypothetical graphs of tree age versus elevation curves. The upper one portrays trees that become gradually younger with elevation gain, much as one might expect if treeline was moving higher gradually. The hypothetical curve on the lower graph describes a more abrupt change, i.e. what one might expect if tree line had been stable for a relatively long period of time, slowly moving to higher elevations, then suddenly and relatively recently, young trees became established higher than before.
But a further question is this: have the young trees in the alpine arrived relatively suddenly and recently because of recent rapid increase in global temperatures, or are they gradually moving upwards due to a long term warming trend that has been taking place ever since the end of the Pleistocene, ca. 13,000 years ago? Dating trees by their growth rings could provide the answer by measuring the ages of trees along an elevation gradient from well below tree-line into the alpine. I suspect this kind of research is underway.
Every year we seem to encounter botanical surprises, either range extensions or species that we haven’t seen before. I like these kinds of discoveries because distribution patterns tell us something about the history of the landscape and when those distribution patterns are found to be different from what was previously known, the background story might change.
One notable collection this year was Dodecatheon frigidum (northern shootingstar) that we collected in northern Graham Laurier Provincial Park, about 200 km south of where it has been collected previously near the Alaska Highway. We’ve visited 8 mountains in the intervening area and have not encountered this species. What does this occurrence mean? Have we merely overlooked it in other areas or is it in fact not present for this 200 km distance?
Another interesting find was Claytonia lanceolata (western spring beauty) which I saw in the alpine for the first time. Previously I had encountered it at lower elevations in Botanie Valley north of Lytton. Indigenous people in the southern interior of BC eat the tubers either fresh or cooked. Perhaps indigenous people in the Tumbler Ridge area also eat the tubers. I haven’t had a chance to ask local people or to investigate the literature.
When is a holotype not a type specimen?
When it was never published in the first place.
The Royal BC Museum fish collection contains a specimen which had been locked securely in one of our type cabinets since the 1980s. It was designated as the holotype for a new species – Sebastes tsuyukii – there was even a manuscript noted on the specimen label (Westreim and Seeb 1989). It sounded legit – and no one checked until recently.
Jody Riley – my ever diligent volunteer – flagged this record when she was re-organising the fish collection. She checked what is in our old paper catalog, checked the electronic database, then looked to see if the actual specimen exists. When Jody hit Sebastes tsuyukii, and found no record of the species online, yet here in her hands was the jar with a big yellow tape label saying Holotype for Sebastes tsuyukii, she knew something was fishy.
 Did the manuscript stall during composition, submission, or revision? Who knows.
Did the manuscript stall during composition, submission, or revision? Who knows.
In the end, we can take this large jar out of the cabinet designated for type specimens, Sebastes tsuyukii now is a nomen nudum (a naked name), and I can delete the species from the taxonomy in our museum database. Some database problems are easy to solve.
But this reminds me to get my fingers in gear and type the type descriptions for species I have yet to publish.
Impact of Facility Renewal Deferment on Risk to Royal British Columbia Museum, Canada, Collections post
Abstract:
The Royal British Columbia Museum (RBCM), Canada, houses a collection of almost 7 million artifacts, archival records, and natural history specimens. Three comprehensive collection risk assessments over the past decade have resulted in improvements to the physical environments of the collections as well as new policies and procedures to reduce risk. However, there remain ongoing risks that can only be mitigated through major facility renewal. The last collection risk assessment, completed in 2016, was revisited to review the data and build a defensible case for funding to replace the RBCM’s on-site collection storage facilities. Changes to overall collections risk is a complex function of collection development and use trends, evolving risk factors both internal and external to the museum, a growing understanding of the relationship between risks and preservation, in addition to reduction due to risk mitigation projects and building systems aging and wearing out. A defensible method for illustrating the facilities-related risks over time involves estimating the expected loss of individual collection items or loss in value of a group of items that may occur if a major facility upgrade or redevelopment is not realized in the near future. Risk assessment data for representative collection units were reviewed to differentiate risk due to permanent facility characteristics versus more active controls, operations budget controlled risk versus capital budget controlled risk, and collection management-controlled risk versus facility management-controlled risk. This enabled the risk model to isolate risks that could only be mitigated through major facility upgrades. Change in collection value was expressed as Object Equivalents Lost (OEL) and its compliment Object Equivalents Remaining (OER). Projections into the future indicating the effect of varying facility renewal dates could then be clearly shown. Losses, when presented as numbers of items expected to be lost from the collection, become emotionally salient to persons in senior management and governance roles.
Yesterday I posted a video from my time up in the mountains in the Tumbler Ridge Global Geopark. Today I give you a video, near that same spot, but from our new Ricoh Theta V 360 camera. Amidst all the botanizing and mushroom-hunting, I was also testing out this new tool for our online learning programs.
If you’ve never watched a 360 video before, use the circular symbol in the top left corner by using your mouse or finger (depending on the device you’re using). If you’ve got Google Cardboard or other VR goggles you can watch the video through that for an enhanced experience. Look up at the sky, look at the ground or at me swiping at bugs and using the camera controls through the app on my phone (not very exciting). Watch for my wave near the end!
Mountain Stream post
My attempt to video a sublime mountain stream without a tripod and while fending off bugs, near Bone Mountain, south of Tumbler Ridge, BC. July 24, 2018
I did not know mushrooms grew at such high elevations, but they do! While up there wandering around at about 6000 feet one of my tasks was to look for mushrooms. Mushrooms in the alpine have not been studied much at all in BC, but mycologist, Dr. Shannon Berch, Research Soil Scientist at the BC Ministry of Environment is on it. For every mushroom I found and photographed, Dr. Ken Marr took a sample, and bottled it to be sent to Dr. Berch for study.
When I’ve been in the alpine in the past, it’s mostly been on long day hikes, where I had to get up and down a mountain during daylight hours. This does not leave much time or energy for wandering around alpine meadows. But on this trip, that is all we did. We walked and walked, looking for plants for the collection. It was easy to lose sight of the others, so I had to keep reminding myself to not wander too far. Thick fogs can come in quickly in the mountains and create a difficult and dangerous situation and although we’d seen no signs of bears, we were also in Grizzly territory.
It was so beautiful up there I just wanted to keep going. Over every rise there was a new vista. There was birdsong, and far in the distance, running water coming from a stream or a waterfall, (I could not tell which but I had to get closer). That’s when (unbidden I swear) dialogue from The Sound of Music, (the part where Maria is being reprimanded by the Mother Superior for being late) came into my head. Clearly, I heard Maria exclaiming how she could never be lost up there, that these were her mountains and besides the birds were singing and the brooks were babbling and she felt like she was being called higher and higher up into the mountains. That was me that day. At one with Maria.
I mentioned my mental imagery to nearby Royal BC Museum botanists Heidi Guest. Heidi in the mountains with (I kid you not) braids in her hair. Being a good sport, Heidi, spontaneously swung her plant collecting gear around her not unlike Maria while singing I have Confidence. I’m telling you it was a moment.
What I was surrounded by up above the treeline:
What I spent most of my time looking at:


Dr. Ken Marr is Curator of Botany at the Royal BC Museum. I ambled with Ken (botanists amble) last month while he was out collecting plants in the mountains south of Tumbler Ridge.
Mountain Excursion – Post #2
Okay, I don’t even know what to tell you about these photos. I only know that there are fossils in every one of them. There are fossils everywhere in the geopark. They were incredibly distracting! I was to be taking 360 video, botanizing and mushroom hunting (more on that in a future post), but kept happening upon these rocky wonders and wanted to just sit down in front of them to look. So I snapped pictures when I could.
Also, we had no paleontologists with us or identification guides. Have a look at the pictures and wonder with me, or if you are in the know, please comment!
Mountain Excursion image
Last wee k I went where few British Columbians go: the high alpine in our spectacular Northern Rockies. When at the last minute one of the Royal BC Museum scientists could not make the trip, I had the good fortune to take her place in the helicopter and spend two days in the field with a small team of museum researchers. My purpose was to bring back photographs and videos for our online learning programs, (especially with our new 360 camera) and to use my naturalist skills to help the botanists collect plants.
k I went where few British Columbians go: the high alpine in our spectacular Northern Rockies. When at the last minute one of the Royal BC Museum scientists could not make the trip, I had the good fortune to take her place in the helicopter and spend two days in the field with a small team of museum researchers. My purpose was to bring back photographs and videos for our online learning programs, (especially with our new 360 camera) and to use my naturalist skills to help the botanists collect plants.
On July 23rd, the helicopter dropped us off about 40 kilometres south of Tumbler Ridge in the Global GeoPark. It was glorious. I took so many photographs I’ve decided to blast you with a series of short photo-posts over the month of August! (It’s like being stuck at a friend’s home movie night, except this is optional and hopefully you won’t feel stuck, but awed by BC’s beautiful and biodiverse northern alpine).

Royal BC Museum field camp tents near an alpine lake.

Alpine flowers including anenomes gone to seed or ‘hippies on a stick’ as they are affectionately and aptly known.
Orca Abscessed post
Nitinat (T12A) was a well known Orca along the BC coast. Born in 1982, he was a fixture along the BC coast and an active participant in the 2002 attack on a Minke Whale in Ganges Harbour, Saltspring Island. This animal – with its characteristically wavy dorsal was found dead off Cape Beale near Bamfield, September 15th, 2016. Funds weren’t available to prepare the entire skeleton, so I had to settle for the skull and jaws.
As you can imagine, the head of an orca would pop the frame of any domestic chest freezer, and it blocked the aisle of the walk-in freezer at the Pacific Biological Station in Nanaimo. It was also no small feat to fork-lift the head into the museum’s van, and then get it back out of the van and wheel it to the museum’s walk-in freezer. It also was a surreal experience driving around with an orca head in the truck. The head is heavy – and slippery – and difficult to tie down – so I drove smoothly to avoid having the head roll around behind me. Imagine explaining to an insurance company how an orca head caused you to lose control of your vehicle?
Nitinat’s head was prepared by Mike DeRoos and Michi Main – their internationally acclaimed business, Cetacea, focuses on cleaning and articulating whale skeletons. While preparing this skull for burial, they noticed that Nitinat had broken teeth. Given that I broke a molar on a frozen Reese’s Piece in a Dairy Queen Blizzard, I could imagine how Biggs Orcas could break a tooth when biting down on a sea lion or elephant seal. Large pinnipeds have dense bones.
Once the skull was cleaned, Mike and Michi found that not only were teeth broken, there also is a nickle-sized hole in the palate and many teeth were abscessed. The hole in the palate is particularly interesting. It has smooth sides and so certainly had healed before Nitinat’s death. Was it a puncture and the source of the infection that caused the distortion of the teeth? Or was it a channel for the abscess to weep into Nitinat’s mouth (not a pleasant thought regardless).
Normal teeth (left) have a long root and recurved crown, with natural wear for their ecotype – but the abscessed teeth were stunning with their broken crown and expanded root. They almost remind me of some squash varieties that are available.
One of the teeth is so swollen that it couldn’t be removed from its distorted socket.
Red lines beside the skull indicate expanded tooth sockets – perhaps age and infection combined to create this effect. The sockets for the abscessed teeth were eroded and far larger than normal sockets (in this non-mammalogist’s opinion). Erik Lambertson made a great scale bar.
Nitinat’s teeth are enough to make anyone who has had a toothache cringe, and a dentist’s eyes pop with fascination. I am just waiting for the day someone requests to see Nitinat as the focus of a pathology research paper. For now, he is a permanent addition to the Royal BC Museum collection and will soon get his official catalog number.
I don’t know if the title of this article is an accurate way to say fork-tailed lizard in German, but the Gabelschwanz-Teufel – the P-38 Lightning (the fork-tailed devil) could take a lot of punishment and still get home at the end of a sortie. A fork-tailed lizard has a parallel story – it has taken a beating and survived.
It is common to find lizards with regenerated tails or tails that are recently dropped – with their tell-tail stump. Sometimes the tip is lost, others about 90% of the tail is lost. The regrown tail segment is never as nice as the original and has different scale patterns and colouration.
This male Wall Lizard photographed by Deb Thiessen, lost its tail near the base and the regenerated tail is obvious. Its meal had a perfect tail.
I have seen fork-tailed, even trident tailed lizards in photos – I remember images like this in the books I poured over earlier in my ontogeny. Had I ever seen one in person? Not until now. During my PhD thesis work, the only fork-tails I thought about were thelodont fishes known from Early Devonian rocks of the Northwest Territories.
This July, Robert Williams, a colleague from University of Leeds in England was here working on Wall Lizards. He was trying to determine if our native Northern Alligator Lizards react in any way to the scent of the European Wall Lizard.
Live animals are not allowed at the Royal BC Museum, so Rob had to perform scent trials in my dining room. The lizards were held in containers in my kitchen – and I thank my wife for her patience.
The work helps give a frame of reference to reactions between the native Sand Lizard in the UK and introduced Wall Lizards, but you’ll have to wait to hear the results. While hunting Wall Lizards on Moss Rocks here in Victoria, Rob caught a fork-tailed specimen.
Since this was such a neat specimen I requested it be saved intact for the Royal BC Museum’s collection. Here is a photo of a fork-tailed Wall Lizard from England, but Rob had to come all the way to the Pacific coast of Canada to catch one.